IHC Troubleshooting
Immunohistochemistry, or IHC, is a complicated process that requires lots of reagents and optimization. Many potential problems can affect the final staining results. Here we have listed some common problems associated with IHC, and the solutions to solve these problems.
? Find IHC Antibodies (browse all)
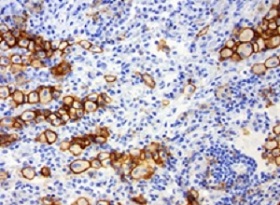
Example of specific IHC staining FFPE human lung cancer stained with anti-PD-L1 antibody (SKU# TA591004).
Common Problems with IHC
Weak Or No Staining
Possible reasons and how to solve this? >
Non-specific Staining
Possible reasons and how to solve this? >
High Background
Possible reasons and how to solve this? >
Weak Or No Staining
| Reasons | Solutions |
|---|---|
| Antibody concentration was too low | Increase the concentration of primary and/or secondary antibodies. |
| Primary antibody not suitable for IHC | Check the antibody datasheet to see if it has been validated for IHC application, and what type of IHC (formalin fixation paraffin embedded, fresh frozen, etc.). |
| Incompatible secondary and primary antibodies | Use a secondary antibody that was raised against the species in which the primary was produced (e.g. primary antibody is raised in mouse, use anti-mouse secondary antibody). |
| Inactive primary and/or secondary antibody | Replace with a new batch of antibody |
| Antibody dissociates from antigen during washing | Use a lower concentration of detergent in washing buffers. Replace with higher affinity antibody or Re-optimize wash steps. |
| The protein of interest is not abundantly present in the tissue | Use an amplification step to maximize the signal. For example, use a biotin conjugated secondary antibody and a conjugated streptavidin to amplify the signal, or use detection kit with high sensitivity, such as GBI Labs Polink-2 HRP polymer detection kit. |
| Improper protocol used for HIER | Refer to recommended Antigen retrieval protocol in antibody insert. |
| Inadequate deparaffinization | Deparaffinize sections longer or change fresh xylene |
| The protein is located in the nucleus and the antibody cannot penetrate the nucleus | Add a strong permeabilizing agent like Triton X to the antibody dilution buffer. Recommend using HIER buffer with detergent. GBI Labs B22C-xx Accel pH 8.7 or B21-xxTEE pH 9.0 |
| Incorrect preparation of substrate-chromogen mixture | Repeat substrate-chromogen treatment with correctly prepared reagent. Staining intensity is decreased when excess DAB/DAB+ is present in the working reagent. |
| The PBS buffer is contaminated with bacteria that damage the phosphate groups on the protein of interest | Add 0.01% sodium azide in the PBS antibody storage buffer or use fresh prepared PBS. |
| The amplification kit may have lost its activity due to improper storage, improper dilution or extensive freezing/thawing | Run positive controls to ensure that the primary/secondary antibody is working properly. |
Non-specific Staining
| Reasons | Solutions |
|---|---|
| Antibody concentration was too high | Try decreasing the antibody concentration and/or the incubation period. |
| Insufficient blocking of endogenous peroxidase or phosphatase | Protocol should always have the step to block endogenous peroxidases and/or phosphatase with 3% H2O2 for peroxidase or 2mM Levanisol for phosphatase. |
| Antibody and tissue from same host species | Use a primary antibody raised against a different species from your tissue. Use kits such as GBI Labs Klear Mouse D52-xx for mouse antibodies on mouse tissues. |
| Serum block step insufficient | Change blocking formula. Goat, horse or donkey serum is interchangeable and will block different background. |
| The sections have partially dried out | Keep sections at high humidity container and do not let them dry out throughout the procedure. |
| Keep sections at high humidity container and do not let them dry out throughout the procedure. | Centrifuge the antibodies at12000g for 5 minutes to get rid of the pellet. Keep and use the supernatant. |
High Background
| Reasons | Solutions |
|---|---|
| Antibody concentration was too high | Titrate the antibody to the optimal concentration, or dilute the antibody further and incubate at 4?C. |
| The secondary antibody cross-reacts with the screening tissue | Run a control with secondary antibody without primary antibody. If you see staining with your secondary only, change to a secondary antibody that has been pre-adsorbed against the immunoglobulin of the species of your samples. |
| Blocking might be absent or insufficient | increase the detergent in the washing buffer, or increase the washing time for each wash step |
| Tissue not washed enough | Titrate the antibody to the optimal concentration, or dilute the antibody further and incubate at 4?C. |
| Amplification ofdetection too strong | Reduce amplification incubation time and dilute the secondary antibody. |
| Too much substrate was applied | Substrate needs to be diluted further, or reduce the substrate incubation time. |
| Permeabilization has damaged the membrane and removed the membrane protein (membrane protein | Use a less stringent detergent (e.g.) Tween 20 instead of Triton X). Or simply remove permeabilizing agent from your buffers. |






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China