Human IL-1a ELISA Kit (96-well)
USD 415.00
In Stock*
Specifications
| Product Data | |
| Format | 96-well strip plate |
| Assay Type | Solid Phase Sandwich ELISA |
| Assay Length | 3 hours |
| Signal | Colorimetric |
| Curve Range | 7.8-500pg/ml |
| Sample Type | Cell culture supernatant, serum, plasma (EDTA, citrate, heparin) |
| Sample Volume | 20 uL |
| Specificity | Natural and recombinant Human IL-1a Ligand |
| Sensitivity | 4pg/mL |
| Reactivities | Human |
| Interference | No significant interference observed with available related molecules. |
| Components |
|
| Background | Interleukin 1 (IL-1) is a name that designates two proteins, IL-1. and IL-1., which are the products of distinct genes, but which recognize the same cell surface receptors. With the exception of skin keratinocytes, some epithelial cells, and certain cells of the central nervous system, IL-1 is not produced by the cells of healthy individuals. However, in response to stimuli such as those produced by inflammatory agents, infections, or microbial endotoxins, a dramatic increase in the production of IL-1 by macrophages and various other cell types is seen. For reviews on the properties and activities of IL-1. and IL-1., see references 1 - 3. IL-1. and IL-1. are structurally related polypeptides that show approximately 25% homology at the amino acid level . Both are synthesized as 31 kDa precursors that are subsequently cleaved into proteins with molecular weights of approximately 17.5 kDa . Neither IL-1. nor IL-1.contains a typical hydrophobic signal peptide sequence , but evidence suggests that these factors can be secreted by non-classical pathways . A large proportion of IL-1. is retained intracellularly in its precursor form . A portion of this unprocessed IL-1. is transported to the cell surface and remains associated with the cell membrane . The membrane-bound, unprocessed IL-1. is apparently biologically active, acting in a paracrine fashion on adjacent cells having IL-1 receptors . The precursor form of IL-1., unlike the IL-1. precursor, shows little or no biological activity in comparison to the 17.5 kDa processed form . Intracellular IL-1.consists exclusively of the 31 kDa precursor form . Extracellular IL-1. consists of a mixture of both unprocessed and mature IL-1.. These results indicate that processing takes place subsequent to secretion and is not tightly coupled to secretion . The specific protease apparently responsible for the processing of IL-1., designated interleukin1.-converting enzyme (ICE), has been described . IL-1. and IL-1. exert their effects by binding to specific receptors. Two distinct receptor types have been isolated that bind both forms of IL-1. An 80 kDa membrane bound receptor protein, IL-1 receptor type I (IL-1 RI), has been isolated from T cells, fibroblasts, keratinocytes, endothelial cells, synovial lining cells, chondrocytes, and hepatocytes . IL-1 RI has been cloned from mouse and human cells and found to be a member of the Ig super family. A second type of IL-1 receptor, IL-1 receptor type II (IL-1 RII), has been found on B cells, neutrophils, and bone marrow cells . This receptor has an apparent molecular weight of about 68 kDa and is also a member of the Ig super family. The two IL-1 receptor types show approximately 28% homology in their extracellular domains, but differ significantly in that the type II receptor has a cytoplasmic domain of only 29 amino acid residues, whereas the type I receptor has a cytoplasmic domain of 213 amino acid residues . In general, IL-1. binds better to the type I receptor and IL-1. binds better to the type II receptor . At present, the mechanisms involved in the transduction of the signal initiated by binding of IL-1 are not well characterized . IL-1 possesses a wide variety of biological activities. It has been shown to induce prostaglandin synthesis in endothelial cells and smooth muscle cells . In the liver, IL-1 initiates the acute phase response resulting in an increase in hepatic protein synthesis and decreased albumin production . |
| Gene Symbol | IL1A |
| Standard Curve |
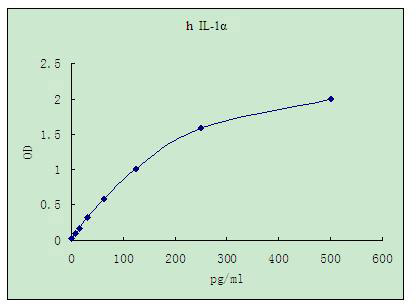
Representative standard curve for IL-1a ELISA. IL-1a was diluted in serial two-fold steps in Sample Diluent.
|
Documents
{0} Product Review(s)
Be the first one to submit a review






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China