SARS-CoV-2 (2019-nCoV) Research Tools
Tools to Investigate SARS-CoV-2 Infection
COVID-19 is caused by the coronavirus SARS-CoV-2, also called 2019-nCoV. The viral spike (S) protein - host cell ACE2 receptor interaction is key to a successful infection of the host cell.
Spike Protein (S): SARS-CoV2 enters the cells through the Spike mediated interaction with the ECD domain of the ACE2 cell receptor. A recombinant fusion protein (RBD of Spike protein and ECD of membrane protein) can a great tool to investigate this interaction.
Learn more about the
purified recombinant Spike Protein (S)
SARS-CoV-2 Spike Mutant Proteins: Recently several SARS-CoV-2 variants with mutation in the spike (S)-protein has been identified. Of all the variants analyzed, variants carrying D614G mutation was significantly more infectious than others. Some of the variants with amino acid change in receptor binding domain (RBD) were less infectious and some were resistant to neutralizing antibodies. Understanding the impact of these mutations is of great value for development of vaccines and therapeutic antibodies.
Key concerned variants:
Y453F
N439K
N501Y
D614G
Full list of SARS-CoV-2 Spike Protein Variants
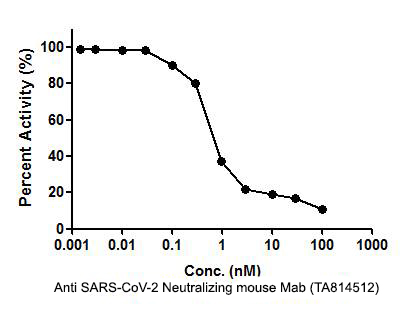
SARS-CoV-2 Neutralizing Antibody: SARS-Cov-2 RBD neutralizing antibody (Cat# TA814512) inhibits human ACE2 protein (Cat#?TP701115) binding to SARS-CoV-2 RBD protein?TP701119). The IC50 is typically 0.91 nM.
Find out more about S Protein RBD and S-Protein? research tools. Try out our

ACE-2 receptor: ACE-2 is the host cell receptor responsible for mediating infection by SARS-CoV-2.
Tools for analyzing ACE-2 protein
- Human Recombinant Protein Angiotensin Converting Enzyme (ACE2)
- ACE-2 specific Mouse Monoclonal Antibody
- ACE-2 Human ORF clones
Find out more about the additional ACE-2 specific tools here






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China