Alzheimer’s Disease
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by dementia that generally initiates with subtle failure of memory, progressing over years to an incapacitating level. Approximately 25 % of all AD is familial of which approximately 95 % is late-onset (age >60-65 years) and 5 % is early-onset (age <60 years).
Learn more about 3 key protein in Alzheimer?s Disease: Amyloid-? (A?) Precursor Protein (APP), Microtubule-associated protein tau (MAPT-Tau), Macrophage scavenger receptor 1 (MSR1).
Key genes associated with Alzheimer?s Disease
APP BACE1 BACE2 PSEN1 PSEN2 UBQLN1 APPBP1 APPBP2 ApoE ApoER2 CLU ACT BDNF CDK5R1 KCNIP1 KCNIP3 MAPT NEFM Bax IL1A IL1B KLK6 MSR1 MEOX2 NQO1Amyloid-? (A?) Peptide
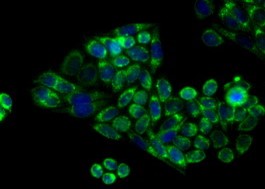
Apolipoprotein E receptor 1 (ApoER2)
The generation of the Amyloid-? (A?) peptide through the proteolytic processing of the Amyloid precursor protein (APP) is a central event in the pathogenesis of AD. Extracellular accumulation of A? leads to formation of aggregates, fibrils and eventually amyloid deposits called neuritic plaques, a hallmark of AD. The A? peptide has neurotoxic effects and AD research has been focused on determining the underlying mechanisms of A? protein toxicity.
Established candidates for the cleavage of APP to A? are the ?-secretases BACE1 and BACE2 and Presenilin 1 and 2, which resemble the catalytic subunit of the ?-secretase complex. PEN2 (presenilin enhancer 2) is the regulatory component of the ?-secretase complex.
APP ApoE ApoER2 APPBP1 APPBP2 BACE1 BACE2 CLU PSEN1 PSEN2 UBQLN1Ubiquilin 1 (UBQLN1) is a ubiquitin-like protein, which has been shown to play a central role in regulating the proteasomal degradation of various proteins, including the presenilins. A role for UBQLN1 steady-state levels in affecting APP trafficking and processing has been proposed, thereby influencing the generation of A?.
Amyloid protein-binding protein 1 (APPBP1) and Amyloid protein-binding protein 2 (APPBP2) interact with APP and are functionally associated with APP transport and/or processing.
Three alleles for Apolipoprotein E (ApoE) are known and there is a strong association of one or two copies of the ApoE allele e4 with late-onset AD, although the mechanism by which this allele impacts AD remains unproven.
(ApoER2) is a member of the LDL receptor family that is highly expressed in the brain. It has been shown that ApoER2 expression stimulates A? production by enhanced ?- and ?-secretase mediated amyloidogenic processing.
Clusterin/Apolipoprotein J (Apo J) has also been identified as a potential risk gene in AD. Elevated protein levels are found in the CNS under some neuropathological conditions, such as AD, where Apo J is associated with A? plaques.
Microtubule-associated protein tau (MAPT-Tau)
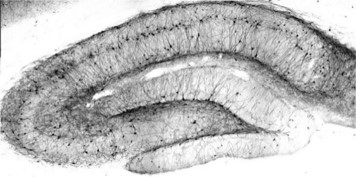
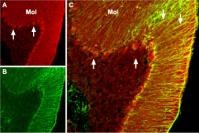
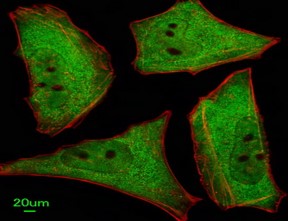
MAPT/Tau is a neuronal microtubule associated protein and it promotes tubulin polymerization and stabilizes microtubules. In its hyperphosphorylated form, tau is the major component of paired helical filaments and neurofibrillary lesions in AD brain. Hyperphosphorylation impairs the microtubule binding function of tau, resulting in the destabilization of microtubules in AD brains, ultimately leading to the degeneration of the affected neurons.
Key genes associated with MAPT/Tau
ACT BDNF CDK5R1 KCNIP1 KCNIP3 MAPT NEFMCyclin-dependent kinase 5 activator 1 (CDK5R1) activates CDK5, which is required for proper development of the central nervous system. CDK5R1 is cleaved from a p35 into a p25 form and has been shown to accumulate in neurons of patients with AD. This accumulation correlates with an increase in CDK5 activity and may lead to aberrantly phosphorylated forms of MAPT/TAU, which contributes to AD.
Alpha 1-antichymotrypsin (ACT) has been shown to promote A? polymerization and levels of ACT protein in plasma and cerebrospinal fluid from Alzheimer?s patients have been found to correlate with progression of dementia. ACT may lead to hyperphosphorylation of tau thereby enhancing degeneration of neurons.
Calsenilin/KCNIP3 and KCNIP1/VABP are members of the family of voltage-gated potassium channel-interacting proteins. They interact with presenilin 1 and 2 and are implicated in the mediation of A? formation.
Brain-derived neurotrophic factor (BDNF) belongs to so-called neurotrophins and supports the survival of existing neurons and encourages the growth and differentiation of new neurons. Neurotrophins are thought to have a protective role against A? toxicity.
Detection of Neurofilament M can be used in studies to visualize neurofilament accumulation as it can be seen in AD.
Macrophage scavenger receptor 1 (MSR1/CD204)
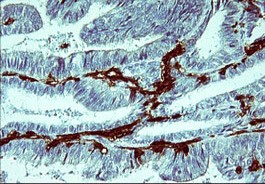
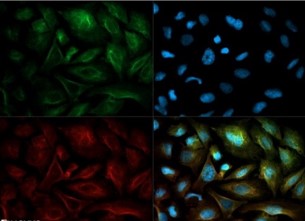
In Alzheimer?s disease, MSR1/CD204 is is increased. Findings of CD204 mediated adhesion and endocytosis of fibrillar A? by microglia and astrocytes suggest a role for this receptor in neuronal homeostasis and neuropathology.
Key genes associated with MSR1/CD204
Bax IL1A IL1B KLK6 MSR1 MEOX2 NQO1Interleukin 1 is a cytokine which is overexpressed in the AD brain. This correlates to plaque formation and progression by leading to excessive expression of neuronal APP and other plaque-associated proteins and to nonsensical growth of dystrophic neuritis. Polymorphism in the IL-1A and IL-1B genes are discussed for early age onset AD.
Kallikrein-6 is a serin protease and abnormal levels have been found in patients with AD. The potential role of Kallikrein-6 as a biomarker for AD is under investigation and it has been reported that this protein might play a role in the degradation of A? or turnover of APP.
Homeobox protein MOX-2 (MEOX2) is a regulator of vascular differentiation and its expression is low in AD. It has been shown that restoring expression of the protein stimulates angiogenesis, suppresses apoptosis and increases the levels of a major A? clearance receptor, the low-density lipoprotein receptor-related protein 1 (LRP), at the blood-brain barrier.
Bax, a pro-apoptotic protein belonging to the Bcl-2 family, promotes increased apoptosis leading to enhanced neuronal degeneration in progression of AD. Bax may play a similar role in Huntington's disease.
NQO1 : While in AD there is abundant evidence for the involvement of oxidative stress, the cause or the consequences are largely unresolved.
NAD(P)H dehydrogenase (quinone 1) (NQO1), a redox-regulated flavoenzyme, plays a central role in monitoring cellular redox state and has been shown to be increased in AD.
References
- Association of CR1, CLU and PICALM with Alzheimer's disease in a cohort of clinically characterized and neuropathologically verified individuals, Corneveaux, JJ, Hum Mol Genet, 2010.
- ApoER2 expression increases A? production while decreasing Amyloid Precursor Protein (APP) endocytosis: Possible role in the partitioning of APP into lipid rafts and in the regulation of ?-secretase activity, Fuentealba, RA,Mol Neurodegener, 2007
- Ubiquilin 1 modulates amyloid precursor protein trafficking and Abeta secretion, Hiltunen, M, J Biol Chem, 2006
- Glia Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system, Husemann, J, 2002
- Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Lambert, J, Nat Genet, 2009
- Humanin and the receptors for humanin, Matsuoka, M, Mol Neurobiol, 2010
- Interleukin-1, neuroinflammation, and Alzheimer's disease, Mrak, RE, Neurobiol Aging, 2001
- Localization of a novel type trypsin-like serine protease, neurosin, in brain tissues of Alzheimer's disease and Parkinson's disease, Ogawa, K, Psychiatry Clin Neurosci, 2000
- Alpha1-antichymotrypsin, an inflammatory protein overexpressed in Alzheimer's disease brain, induces tau phosphorylation in neurons, Padmanabhan, P, Brain, 2006
- Quinone reductase (NQO1), a sensitive redox indicator, is increased in Alzheimer's disease, Raina AK, Redox Rep., 1999
- The neuronal cyclin-dependent kinase 5 activator p35Nck5a and Cdk5 activity in monocytic cells, Studzinski, GP and Harrison JS, Leuk Lymphoma, 2003
- Alzheimer?s disease: relevant molecular and physiopathological events affecting amyloid-? brain balance and the putative role of PPARs, Zolezzi, JM, Front Aging Neurosci, 2014






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China