M1 and M2 Macrophage Markers
What are macrophages?
Macrophages are phagocytic cells of the immune system that play a pivotal role in orchestrating the innate immune response. They originate from circulating monocytes, which upon activation by pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) within the microenvironment, undergo a series of morphological and functional changes to transform into mature macrophages.Macrophages are endowed with a remarkable repertoire of effector functions, which enable them to eliminate invading pathogens efficiently. These include phagocytosis, antimicrobial peptide secretion, reactive oxygen, nitrogen species production, and cytokine and chemokine secretion. Notably, macrophages also possess antigen-presenting capabilities and can present antigenic peptides to T cells, thereby initiating the adaptive immune response.
Beyond their immune-related functions, macrophages also play a central role in tissue homeostasis and repair. Macrophages can promote angiogenesis and granulation tissue formation during wound healing and facilitate tissue regeneration by producing growth factors and interacting with stem cells.However, dysregulated macrophage activity has been implicated in the pathogenesis of several chronic inflammatory disorders, such as atherosclerosis, rheumatoid arthritis, and neurodegenerative diseases. In these conditions, persistent stimuli may activate macrophages and produce excessive pro-inflammatory cytokines, leading to tissue damage and dysfunction.Overall, macrophages are a highly versatile cell type critical to innate and adaptive immune response, tissue repair, and regeneration. Further elucidation of the molecular mechanisms regulating macrophage function may lead to the developing of novel therapeutics for treating inflammatory diseases.
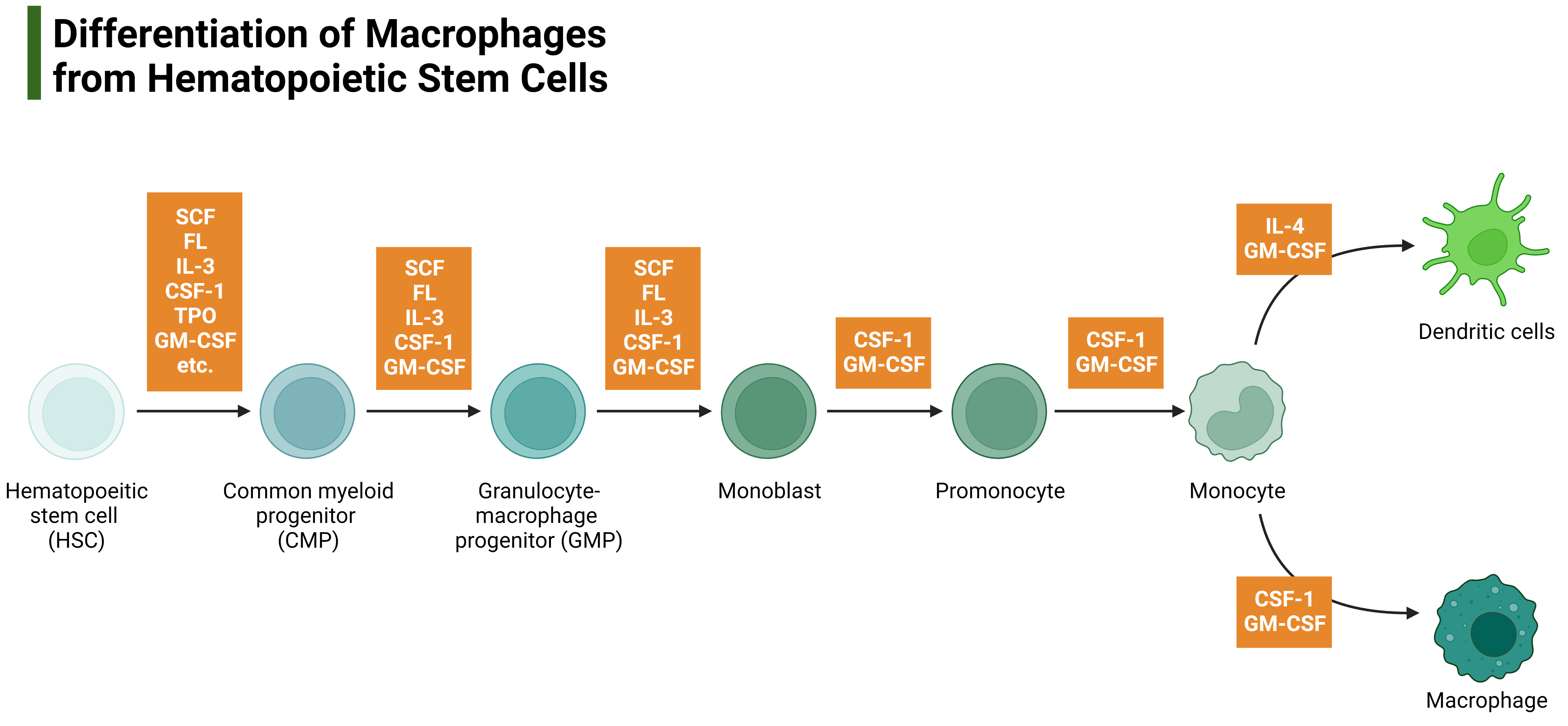
Fig1: Differentiation of Macrophages
Types of Macrophages ?(M1 and M2)
Macrophages are a highly diverse cell population that can assume different phenotypes and functions in response to local environmental cues. Here are some of the main types of macrophages:
1. Classically activated macrophages (M1): These macrophages are induced by interferon-gamma (IFN-?) and/or microbial stimuli such as lipopolysaccharide (LPS). M1 macrophages exhibit a pro-inflammatory phenotype and produce cytokines such as tumor necrosis factor-alpha (TNF-?), interleukin-1 beta (IL-1?), and IL-6, critical for host defense against pathogens.
2. Alternatively activated macrophages (M2): These macrophages are induced by interleukin-4 (IL-4), IL-13, or IL-10, and are typically associated with tissue repair and remodeling. M2 macrophages produce anti-inflammatory cytokines such as IL-10 and transforming growth factor-beta (TGF-?) and extracellular matrix proteins that facilitate tissue repair.
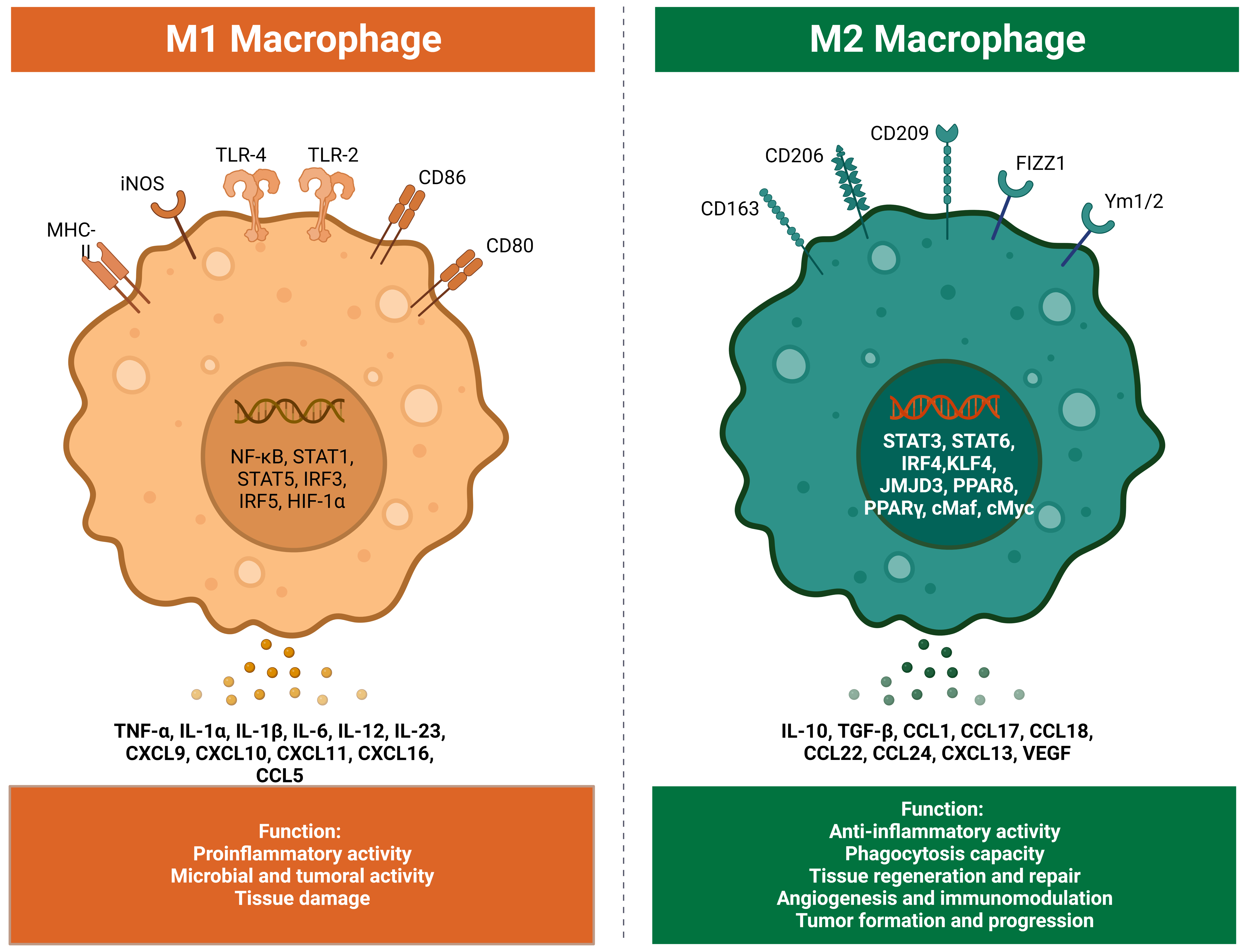
Fig 2: Different types of macrophages
What is the difference between M1 and M2 macrophages?
M1 and M2 macrophages are two phenotypically distinct subpopulations. M1 macrophages are classically activated macrophages that exhibit a pro-inflammatory phenotype characterized by the production of high levels of cytokines such as IL-1?, IL-6, and TNF-?. These cytokines initiate and propagate inflammation and are critical for clearing intracellular pathogens such as bacteria and viruses. M1 macrophages also express high levels of surface markers, such as CD86 and MHC class II, which are associated with antigen presentation and T cell activation. Furthermore, M1 macrophages rely on glycolysis for their energy metabolism and produce high levels of ROS, which are essential for killing intracellular pathogens.
On the other hand, M2 macrophages are alternatively activated macrophages that exhibit an anti-inflammatory phenotype characterized by the production of cytokines such as IL-10 and TGF-?, which are involved in the resolution of inflammation and tissue repair. M2 macrophages also express high levels of surface markers such as CD163 and CD206, which are associated with tissue repair and immunosuppression. In contrast to M1 macrophages, M2 macrophages rely on oxidative phosphorylation for their energy metabolism and produce lower levels of ROS.
The balance between M1 and M2 macrophages is critical for maintaining tissue homeostasis and preventing excessive inflammation and tissue damage. Dysregulation of this balance has been implicated in the pathogenesis of various diseases, including chronic inflammation, cancer, and autoimmune disorders. Therefore, understanding the mechanisms that regulate the switch between M1 and M2 phenotypes is of great interest for developing therapeutic interventions that target macrophages in disease states.
Subset of M1 and M2 Macrophages
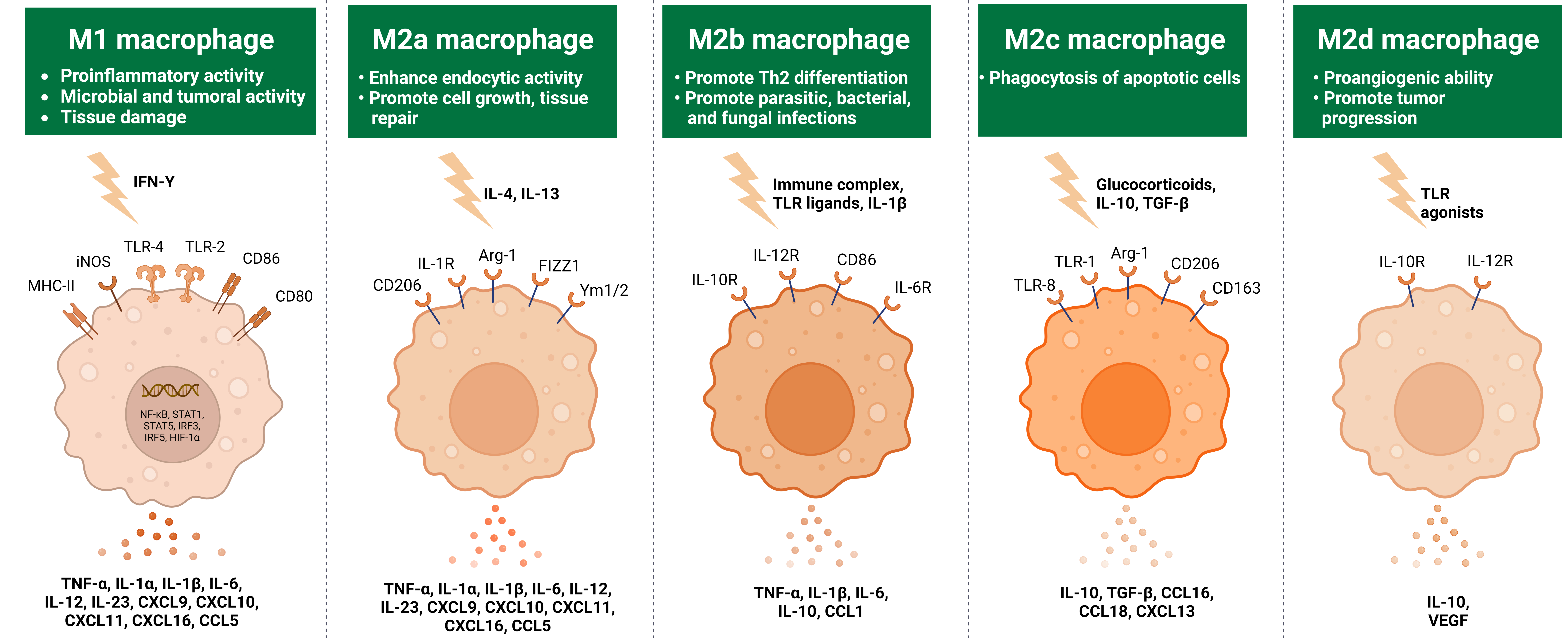
Fig 3: Subset of M1 and M2 macrophages
Antibodies to Analyze M1 & M2 Macrophage Subsets
| M1 | M2a | M2b | M2c | M2d | |
|---|---|---|---|---|---|
| Stimulation/Activation | IFN-gamma | IL-4 | IL-1B | IL-10 | IL-6 |
| LPS | IL-13 | IL-1R | TGF-beta | Adenosine | |
| Marker Expression | CD86 | CD163 | CD86 | CD163 | VEGF |
| CD80 | MHC II | ||||
| CD68 | SR | MHC II | TLR8 | ||
| MHC II | CD206 | ||||
| IL-1R | CD200R | TLR1 | |||
| TLR2 | TGM2 | IL-10R | |||
| TLR4 | DecoyR | Arg-1 | |||
| iNOS | IL-1R II | ||||
| SOCS3 | FIZZ1 | IL-12R | CD206 | ||
| Arg-1 | |||||
| Cytokine secretion | TNF | IL-10 | IL-1 | IL-10 | IL-10 |
| IL-1 beta | TGF-beta | IL-6 | IL-12 | ||
| IL-1 alpha | IL-1ra | TNF-alpha | TNF-alpha | ||
| IL-6 | IL-6 | TGF-beta | |||
| IL-12 | IL-12 | IL-10 | TGF-beta | ||
| IL-23 | IL-23 | ||||
| Chemokine secretion | CCL10 | CCL17 | CCL1 | CCR2 | CCL5 |
| CCL11 | CCL22 | ||||
| CCL5 | CCL24 | CCL13 | |||
| CCL8 | CCL10 | CXCL10 | |||
| CCL16 | CCL11 | CCL18 | |||
| CCL9 | CCL5 | ||||
| CCL2 | CCL16 | CXCL16 | |||
| CCL3 | CCL16 | ||||
| CCL4 |
Table 1: Tools to analyze M1 and M2 macrophages subset
Dual role of Macrophages
Macrophages, play a dual role in the tumor microenvironment, exhibiting both pro-tumor and anti-tumor properties. The tumor-associated macrophages (TAMs), a subpopulation that infiltrate the tumor microenvironment, can promote tumor growth and progression through various mechanisms. For instance, TAMs can produce growth factors and cytokines, such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and IL-10, that stimulate angiogenesis, tumor cell proliferation, and invasion. Additionally, TAMs can suppress the adaptive immune response by producing immunosuppressive cytokines, such as IL-10 and transforming growth factor-beta (TGF-?), that inhibit T cell activation and function. Moreover, TAMs can create a favorable tumor microenvironment that supports tumor cell survival and proliferation by producing extracellular matrix proteins, such as collagen, that protect tumor cells from apoptosis.
In contrast, macrophages can also exert anti-tumor effects in the tumor microenvironment. For example, macrophages can participate in immune-mediated tumor suppression by promoting antigen presentation, T cell activation, and the production of pro-inflammatory cytokines, such as IFN-?, that stimulate T cell-mediated tumor killing. Moreover, macrophages can recruit and activate other immune cells, including natural killer cells and dendritic cells, contributing to the anti-tumor immune response.
The complex interplay between pro-tumor and anti-tumor functions of macrophages in the tumor microenvironment is regulated by various factors, including the type and stage of the tumor and the host immune status. Therefore, gaining insights into the underlying mechanisms that control macrophage function in the tumor microenvironment is crucial for developing effective therapeutic interventions targeting macrophages in cancer. Notably, approaches that aim to reprogram TAMs towards an anti-tumor phenotype, such as by promoting macrophage polarization towards an M1-like phenotype or enhancing macrophage-mediated antigen presentation and T cell activation, hold great potential for cancer immunotherapy.
Key macrophage biomarkers in tumor microenvironment
CD47 IDO Adenosine IL-41 TGF-B TLR STING CD40 CD39Signaling molecules involved in M1/M2 Polarization
A complex network of transcription factors and signaling molecules regulate the differentiation of macrophages into M1 or M2 phenotypes. M1 macrophage polarization is driven by the transcription factors interferon regulatory factor 5 (IRF5) and nuclear factor-kappa B (NF-?B). Upon activation by IFN-? or LPS, IRF5 and NF-?B translocate to the nucleus and promote the expression of pro-inflammatory cytokines, such as IL-1?, IL-6, and TNF-?. Additionally, STAT1 and STAT6 transcription factors are also involved in M1 polarization. STAT1 is activated by IFN-? and promotes the expression of inducible nitric oxide synthase (iNOS), a hallmark of M1 macrophages.
M2 macrophage polarization is driven by transcription factors such as STAT6, peroxisome proliferator-activated receptor gamma (PPAR-?), and Kr?ppel-like factor 4 (KLF4). IL-4 and IL-13 activate STAT6, which promotes the expression of M2-associated genes, such as arginase 1 (ARG1), mannose receptor (MRC1), and chitinase 3-like 3 (CHI3L3). PPAR-? is also involved in M2 polarization, and its activation promotes the expression of M2-associated genes, including IL-10 and TGF-?. KLF4, a zinc finger transcription factor, can be induced by IL-4 and IL-13 and is essential for M2 polarization by promoting the expression of M2-associated genes.
Signaling molecules such as JAK/STAT, PI3K/Akt, and MAPK pathways are also involved in M1 and M2 polarization. The JAK/STAT pathway is activated by cytokines such as IFN-? and IL-4 and promotes the activation of STAT1 and STAT6, respectively. The PI3K/Akt pathway is activated in M2 macrophages and promotes M2-associated gene expression through PPAR-?. The MAPK pathway, including ERK, JNK, and p38, is activated in M1 macrophages and promotes the expression of pro-inflammatory cytokines.
M1 Macrophage Markers
CD80: CD80, also known as B7-1, is a co-stimulatory molecule expressed by antigen-presenting cells (APCs) like macrophages. It interacts with CD28 and cytotoxic T lymphocyte antigen 4 (CTLA-4) on T cells to regulate T cell activation and tolerance. CD80 expression is upregulated on activated macrophages, which polarize into M1 phenotype, producing pro-inflammatory cytokines and phagocytose pathogens. Although M1 macrophages do not exclusively express CD80, it is widely used as a marker of M1 polarization and other markers such as CD86, inducible nitric oxide synthase (iNOS), and tumor necrosis factor-alpha (TNF-?).
CD32: CD32, or Fc?RII, is a low-affinity receptor for the Fc region of immunoglobulin G (IgG) molecules. CD32 is expressed on the surface of various immune cells, including macrophages, dendritic cells, and B cells. CD32 has two isoforms, CD32a, and CD32b, which differ in their intracellular domains and signaling properties. While CD32 expression has been associated with M1 and M2 macrophage phenotypes, some studies suggest that CD32 may be preferentially expressed in M1 macrophages. For instance, a study by Wong et al. (2016) found that CD32 expression was upregulated on M1-like macrophages stimulated with LPS and IFN-?, compared to M2-like macrophages stimulated with interleukin-4 (IL-4) and IL-13.
iNOS: iNOS, also known as nitric oxide synthase 2 (NOS2), is an enzyme that plays a key role in the production of nitric oxide (NO) in response to various stimuli, including pro-inflammatory cytokines such as IFN-? and LPS. NO produced by iNOS is involved in host defense against pathogens and has various other physiological functions.iNOS expression is a well-known marker of M1 macrophages.
CD68: CD68 is a transmembrane glycoprotein primarily expressed on the surface of macrophages and monocytes. It is also found in other cells, including microglia, Kupffer cells, and dendritic cells. CD68 is involved in the phagocytosis of foreign particles and is a marker of mature macrophages. While CD68 is not a specific marker for M1 macrophages, its expression has been upregulated in M1 macrophages compared to M2 macrophages.
CD11b: CD11b, also known as integrin alpha M (ITGAM), is a surface receptor that is primarily expressed on the surface of myeloid cells, including monocytes, macrophages, and neutrophils. CD11b plays a role in cell adhesion, migration, and phagocytosis.
CD64: CD64, also known as Fc?RI, is a high-affinity receptor for the Fc region of immunoglobulin G (IgG) molecules. CD64 expression is upregulated on M1 macrophages in response to inflammatory stimuli such as lipopolysaccharide (LPS) and interferon-gamma (IFN-?).
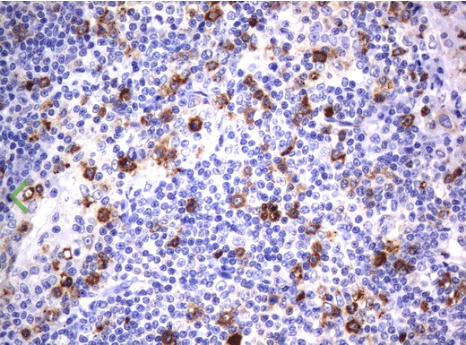
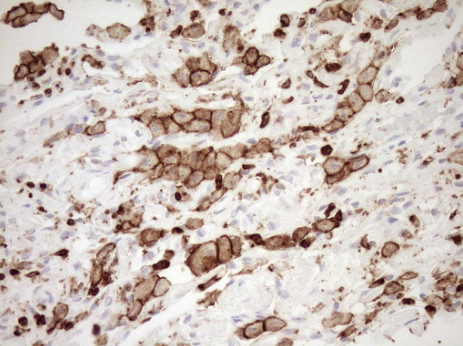
Immunohistochemical staining of paraffin-embedded Human lymphoma tissue using anti-CD80 mouse monoclonal antibody. (UM500055; heat-induced epitope retrieval by 10mM citric buffer, pH6.0, 120?C for 3min)
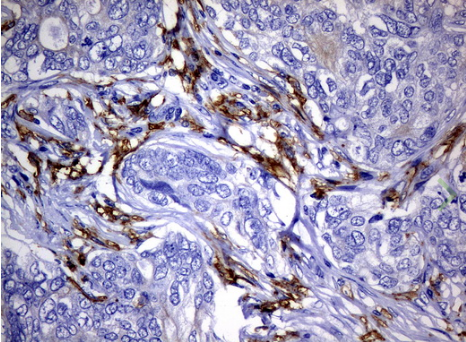
Immunohistochemical staining of paraffin-embedded Adenocarcinoma of Human breast tissue using anti-FCGR2A mouse monoclonal antibody. (UM500051; heat-induced epitope retrieval by 10mM citric buffer, pH6.0, 120?C for 3min)
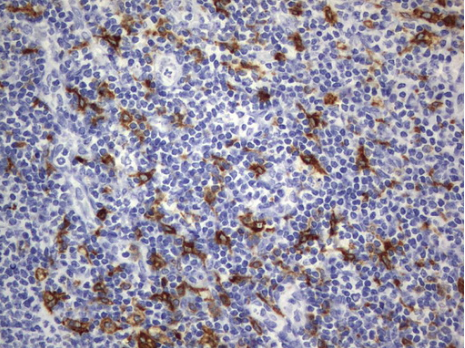
Immunohistochemical staining of paraffin-embedded Human lymph node tissue using anti-CD68 mouse monoclonal antibody. (UM800047; heat-induced epitope retrieval by 10mM citric buffer, pH6.0, 120?C for 3min)
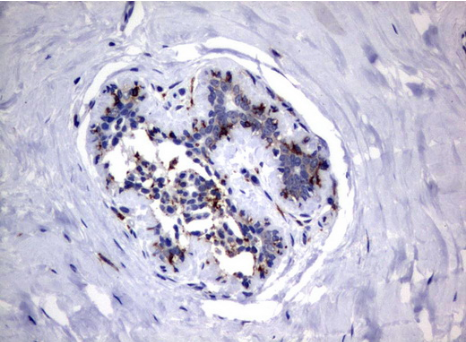
Immunohistochemical staining of paraffin-embedded Human breast tissue using anti-FCGR1A mouse monoclonal antibody. (UM500062; heat-induced epitope retrieval by 10mM citric buffer, pH6.0, 120?C for 3min) (1:100)
Immunohistochemical staining of paraffin-embedded Human lung tissue within the normal limits using anti-ITGAM mouse monoclonal antibody. (Heat-induced epitope retrieval by 1mM EDTA in 10mM Tris buffer (pH8.5) at 120?C for 3min,?TA807953) (1:150)
M1 Macrophage Antibody sampler Kit ? customizable
M2 Macrophage Markers
CD206: CD206, also known as mannose receptor C type 1 (MRC1), is a membrane-bound protein primarily expressed on the surface of macrophages. CD206 is one of the most commonly used markers for identifying M2 macrophages, a subtype of macrophages that play a critical role in tissue repair and anti-inflammatory responses.
CD163: CD163 is another commonly used marker for identifying M2 macrophages and is also known as a scavenger receptor for the hemoglobin-haptoglobin complex. CD163 is a transmembrane protein predominantly expressed on the surface of monocytes and macrophages. M2 macrophages express high levels of CD163 and other M2-associated markers such as CD206 and arginase-1. CD163 has been shown to play a role in the regulation of inflammation, as well as in the clearance of hemoglobin and the prevention of oxidative stress.
CD68: CD68 is a transmembrane glycoprotein primarily expressed on the surface of macrophages and is commonly used as a general marker for macrophages. While CD68 is not a specific marker for M2 macrophages, it can be expressed on M1 and M2 macrophages and other myeloid cells.
ARG1: ARG1, also known as arginase-1, is a critical enzyme in the urea cycle responsible for L-arginine metabolism. ARG1 is predominantly expressed in the liver and in various other tissues and cell types, including macrophages. ARG1 expression is induced by the M2-associated cytokines, including interleukin-4 (IL-4), interleukin-10 (IL-10), and transforming growth factor-beta (TGF-beta). ARG1 expression is often used in combination with other M2-associated markers, such as CD206 and CD163 to identify and characterize M2-like macrophages.
CD11b: CD11b, or integrin alpha M, is a transmembrane glycoprotein primarily expressed on the surface of myeloid cells, including macrophages. CD11b involves cell adhesion, migration, phagocytosis, and immune response. While CD11b is not a specific marker for M2 macrophages, it is expressed on M1 and M2 macrophages and other myeloid cells. The expression of CD11b can vary depending on the specific tissue or disease context and is often used in combination with other markers, such as CD206 and CD163, to identify and characterize M2 macrophages.
MSR1:MSR1, also known as macrophage scavenger receptor 1 or SR-A, is a type I transmembrane protein primarily expressed on the surface of macrophages. It recognizes and uses various ligands, including modified lipoproteins, bacterial products, and apoptotic cells. Various stimuli, including oxidized lipids, cytokines, and bacterial products, induce MSR1 expression. MSR1 expression is associated with an M2-like phenotype in some contexts, as it is upregulated on M2-like macrophages in response to IL-4 stimulation.
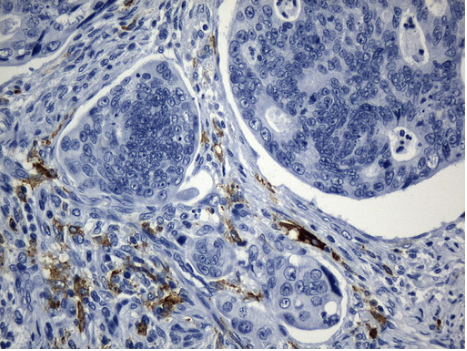
Immunohistochemical staining of paraffin-embedded Adenocarcinoma of Human colon tissue using anti-MSR1 mouse monoclonal antibody. (Heat-induced epitope retrieval by 1mM EDTA in 10mM Tris buffer (pH8.5) at 120?C for 3min, UM800139) (1:1200)
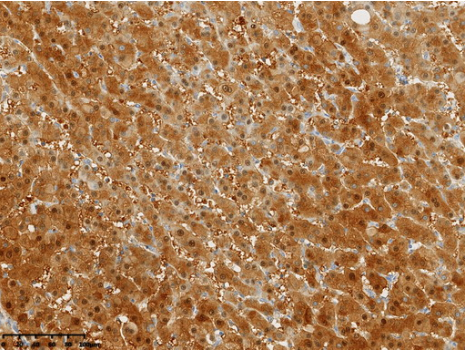
Immunohistochemical staining of paraffin-embedded Carcinoma of Human liver tissue using anti-Arginase-1(ARG1) mouse monoclonal antibody. (Heat-induced epitope retrieval by 1mM EDTA in 10mM Tris buffer (pH8.0) at 120?C for 3 min,?UM800178) (1:15000)
Immunohistochemical staining of paraffin-embedded Human tonsil within the normal limits using anti-CD163 mouse monoclonal antibody. (Heat-induced epitope retrieval by 1mM EDTA in 10mM Tris buffer (pH8.5) at 120?C for 3min, TA506386) (1:500)
Immunohistochemical staining of paraffin-embedded Human lymph node tissue using anti-CD68 mouse monoclonal antibody. (UM800047; heat-induced epitope retrieval by 10mM citric buffer, pH6.0, 120?C for 3min)
Immunohistochemical staining of paraffin-embedded Human lung tissue within the normal limits using anti-ITGAM mouse monoclonal antibody. (Heat-induced epitope retrieval by 1mM EDTA in 10mM Tris buffer (pH8.5) at 120?C for 3min,?TA807953) (1:150)
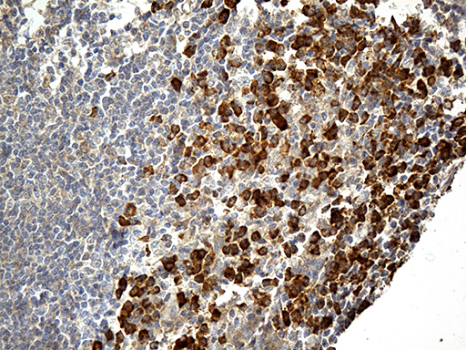
Frozen Human Tonsil stained with Mouse anti Human CD206 antibody (Clone 7-450).
M2 Macrophage Antibody Panel
All Macrophage marker and tools to analyze them
| Gene | Marker Type | Localization |
|---|---|---|
| EMR1 | Pan, Tissue | Cell Membrane |
| CD192 | Monocyte, TAM | Cell Membrane |
| CD14 | Pan, Tissue (cardiac) | Cell Membrane |
| CD68 | Pan, Monocyte, Tissue (Kupffer, alveolar, interstitial, marginal zone, metaophillic, white pulp) | Cell Membrane |
| CD115 | Pan, M2, Monocyte, TAM | Cell Membrane |
| Ly6c1 | Pan, Monocyte | Cell Membrane |
| MARCO | M1, TAM, Tisue (alveolar, marginal zone) | Cell Membrane |
| CD206 | M2, Tissue (alveolar, dermal, red pulp) | Cell Membrane |
| iNOS | M1 | Cytoplasm |
| PPARG | M2, Tissue (alveolar, adipose-associated) | Nucleus, Cytoplasm |
| CD169 | Tissue (bone marrow, Kupffer, dermal, metalophilic, alveolar) | Cell Membrane, Secreted |
| CD282 | M1 | Cell Membrane, Cytoplasm |
| ARG1 | M2 | Cytoplasm |
| CD163 | M2, Monocyte, Tissue (perivascular, Kupffer) | Cell Membrane |
| CD200R1 | Tissue (alveolar) | Cell Membrane, Secreted |
| CD80 | M1, Tissue (liver) | Cell Membrane |
| CD86 | M1 | Cell Membrane |
| CD301 | M2, Tissue (dermal) | Cell Membrane |
| CD369 | M2, Tissue (alveolar, dermal) | Cell Membrane, Cytoplasm |
| CSF2 | M1, Tissue (alveolar) | Secreted |
| CX3CR1 | Pan, Monocyte, Tissue (intestinal) | Cell Membrane |
| CD64 | Pan, Tissue (intestinal, cardiac) | Cell Membrane |
| CD11b | Pan, Monocyte, Tissue | Cell Membrane |
| MERTK | Pan, Tissue (cardiac) | Cell Membrane |
| Pdl1 | M2, TAM | Cell Membrane |
| Retnla | M2 | Secreted |
| TNFA | M1 | Cell Membrane, Secreted |
| CCL22 | M2 | Secreted |
| CD36 | Other | Cell Membrane |
| CD40 | TAM | Cell Membrane, Secreted |
| IL10 | M2 | Secreted |
| IL1B | M1 | Cell Membrane, Cytoplasm, Secreted |
| IL6 | M1 | Secreted |
| LGALS3 | Tissue (Kupffer, alveolar) | Nucleus, Cytoplasm, Secreted |
| MHCII | Pan, M1, M2 | Cell Membrane |
| CD284? | M1 | Cell Membrane |
| CCL2 | TAM | Secreted |
| CD195 | Pan | Cell Membrane |
| CD209 | Tissue (marginal zone) | Cell Membrane |
| CD63 | Other | Cell Membrane |
| CD86 | M1 | Cell Membrane |
| CSF1 | TAM | Cell Membrane |
| CXCL2 | M1 | Secreted |
| CD16 | Pan, M2, Monocyte, TAM | Cell Membrane, Secreted |
| IFNG | M1 | Secreted |
| IL4 | M2 | Secreted |
| IRF4 | M2 | Nucleus |
| ITGAX | Tissue (intestinal, alveloar, peritoneal, Langerhans) | Cell Membrane |
| CD204 | Pan | Cell Membrane |
| PDGFB | M2,TAM | Secreted |
| CD45 | Tissue (adipose-associated, perivascular, meningeal) | Cell Membrane |
| STAT6 | M2 | Nucleus, Cytoplasm |
| TIMD4 | Tissue | Cell Membrane |
| Chil3 | M2 | Nucleus, Secreted |
| CLEC6A | Tissue (dermal, marginal zone, red pulp) | Cell Membrane |
| IL1R1 | M1 | Cell Membrane |
| CD18 | Other | Cell Membrane |
| PDCD1LG2 | M2, TAM | Cell Membrane |
| TLR7 | TAM | Cell Membrane, Cytoplasm |






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China