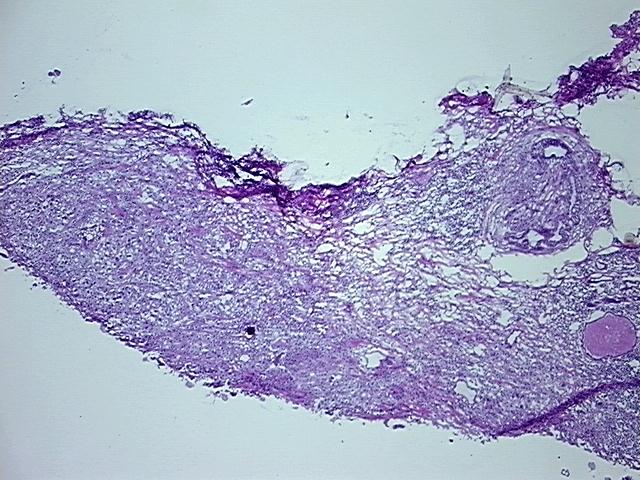
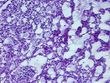
Tissue FFPE Sections, Breast
CAT#: CS555577
Tissue Sections (5x5um), FFPE; Breast; Adenocarcinoma of breast, ductal
USD 380.00
2 Weeks*
USD 331.00
USD 179.00
Specifications
| Product Data | |
| Disease State | Cancer |
| Diagnosis Category | Breast Cancer |
| Tissue | Breast |
| Frozen Sample ID | FR5B3434EC |
| Case ID | CI0000021403 |
| Tissue of Origin | Breast |
| Site of Finding | Breast |
| Appearance | T |
| Sample Diagnosis (from Pathology Verification) | Adenocarcinoma of breast, ductal |
| Normal | 85% |
| Lesional | 0% |
| Tumor | 14% |
| Tumor Hypo/Acellular Stroma | 1% |
| Tumor Hypercellular Stroma | 0% |
| Necrosis | 0% |
| Pathology Verification notes from H&E review | Inflammation: Moderate Lymphoid infiltrate; Non Tumor Structures: 20% Adipose tissue, 30% Glandular epithelium, 50% Stroma; Other Features/Comments: Invasive ductal carcinoma and DCIS: 2 mm x 1 mm each |
| CASE Diagnosis (from medical center path report) | Adenocarcinoma of breast, ductal |
| Age | 66 |
| Gender | Female |
| Race | White or Caucasian |
| Tumor Grade | Nottingham G2: 6-7 points Intermediate combined grade (moderately favorable) |
| Minimum Stage Grouping | IIIA |
| T (Extent of Primary Tumor) | T3 |
| N (Lymph node metastasis) | N2 |
| M (Distant metastasis) | MX |
| Diagnostic Test Results | Progesterone receptor ~ PR ! by IHC,Negative; Estrogen receptor ~ ER ! by IHC,Negative; ERBB2,Amplified copy number |
Documents
| SDS |
{0} Product Review(s)
Be the first one to submit a review






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China