CD15 Mouse Monoclonal Antibody [Clone ID: BRA-4F1]
Specifications
| Product Data | |
| Clone Name | BRA-4F1 |
| Applications | FC, IF |
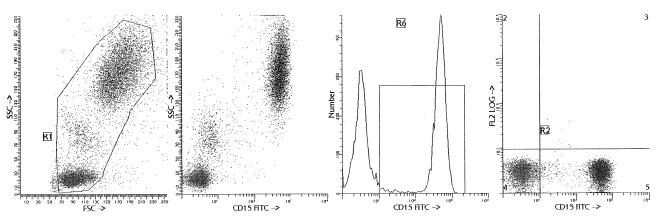
| Recommended Dilution | Suitable for studies of activated T cells, analysis of myeloid leukemias, myeloid differentiation and identification of Reed-Sternberg cells in subtypes of Hodgkin’s disease. - Flow cytometry: for analysis of blood and bone marrow samples. The reagent is effectively formulated for direct immunofluorescent staining (see "Protocols" below). - Immunofluorescence: using cytospots or frozen tissue sections. |
| Reactivities | Human |
| Host | Mouse |
| Isotype | IgM |
| Clonality | Monoclonal |
| Specificity | Clone BRA-4F1 is specific for the Lewis x antigen. It does not cross react with the sialylated form of CD15. |
| Formulation | 0.01 M sodium phosphate, 0.15 M NaCl, pH 7.3, 0.2% BSA, 0.09% sodium azide Label: FITC State: Liquid purified Ig fraction |
| Purification | Affinity chromatography |
| Conjugation | FITC |
| Background | CD15 is expressed on neutrophils, eosinophils and monocytes, but not on platelets, erythrocytes, normal B and T cells. It is also present in embryonic tissues and adenocarcinomas, myeloid leukemias and Reed-Sternberg cells [1,2]. CD15 antibodies recognize the terminal trisaccharide structure which is also referred to as the Lewis x antigen. This structure is found on a variety of glycoproteins and glycolipids at the cell surface [3,4]. CD15 antibodies have been shown to affect a number of cell activities, but it is difficult to distinguish between effects on the CD15 structure itself and effects mediated by proteins which happen to carry the CD15 epitope. CD15 antibodies can mediate complement activation and may have potential therapeutic value in the killing of CD15-expressing tumor cells [2]. |
| Synonyms | Lewis X, X-Hapten, Lacto-N-Fucopentaose III, Stage-Specific Embryonic Antigen, SSEA1 |
| Note | 1. Conjugates with brighter fluorochromes, like PE and APC, will have a greater separation than those with dyes like FITC. When populations overlap, the percentage of positive cells using a selected marker can be affected by the choice of fluorescent label. 2. Use of monoclonal antibodies in patient treatment can interfere with antigen target recognition by this reagent. This should be taken into account when samples are analyzed from patients treated in this fashion. 3. Reagent data performance is based on EDTA-treated blood. Reagent performance can be affected by the use of other anticoagulants. Protocol: Flow cytometry method for use with labeled (FITC, R-PE, APC, PerCP or PerCP-Cy5.5) monoclonal antibodies 1. Add 100 µl of EDTA-treated blood (i.e. approx. 10e6 leukocytes) to a 5 ml reagent tube. The content of one tube is sufficient to perform one test. 2. Add to each tube 10 µl of labeled monoclonal antibody. (Appropriate mouse Ig isotype control samples should always be included in any labeling study). Vortex the tube to ensure thorough mixing of antibody and cells. 3. Incubate the tube for 15 minutes at room temperature in the dark. 4. Add 100 µl of a lyse reagent. 5. Incubate for 10 minutes at room temperature in the dark. 6. Add 2 ml of demineralized water and incubate for 10 minutes in the dark. 7. Centrifuge the labeled cell suspension for 2 minutes at 1000 x g. 8. Remove the supernatant and resuspend the cells in 200 µl of PBS. 9. Analyze by flow cytometry within four hours (alternatively, the cells may be fixed by 0.05% of formaline in buffered saline for analysis the next day. Some antigens are readily destroyed upon fixation and this should be taken into account when using this alternative). Flow cytometry method for use with dual and triple combinations 1. Add 100 µl of EDTA-treated blood (i.e. approx. 10e6 leukocytes) to a 5 ml reagent tube. The content of one tube is sufficient to perform one test. For combinations with anti-kappa and/or anti-lambda Ig see Application note below. 2. Add to each tube 20 µl of labeled monoclonal antibody combination. (Appropriate mouse Ig isotype control samples should always be included in any labeling study). 3. Vortex the tube to ensure thorough mixing of antibody and cells. 4. Incubate the tube for 15 minutes at room temperature in the dark. 5. Add 100 µl of a lyse reagent and mix immediately. 6. Incubate for 10 minutes at room temperature in the dark. 7. Add 2 ml of demineralized water and incubate for 10 minutes in the dark. 8. Centrifuge the labeled cell suspension for 2 minutes at 1000 x g. 9. Remove the supernatant and resuspend the cells in 200 µl of PBS. 10. Analyze by flow cytometry within four hours (alternatively, the cells may be fixed by 0.05% of formaline in buffered saline for analysis the next day. Some antigens are readily destroyed upon fixation and this should be taken into account when using this alternative). Application note for anti-kappa and/or anti-lambda Ig combinations Add 2 ml of PBS containing 0.001% (v/v) Heparin (prewarmed to 37°C) to the cell suspension Vortex, centrifuge (2 min at 300x g) and discard the supernatant. Repeat this step twice. Resuspend the pelleted blood cells in 100 µl PBS, pH 7.2, containing 0.001% (v/v) Heparin. |
| Reference Data | |
Documents
| Product Manuals |
| FAQs |
| SDS |
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China