CD59 Mouse Monoclonal Antibody [Clone ID: NaM172-2B5]
Other products for "CD59"
Specifications
| Product Data | |
| Clone Name | NaM172-2B5 |
| Applications | FC, IF, IHC |
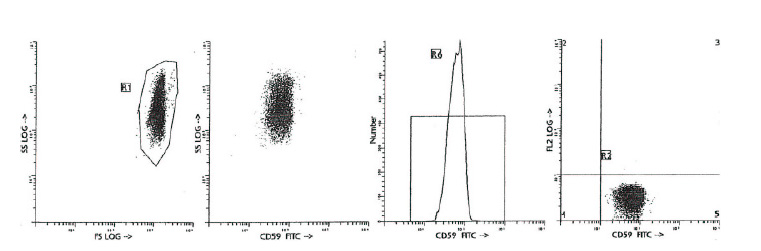
| Recommended Dilution | CD59 antibody clone NaM172-2B5 can be applied in flow cytometry for analysis of blood and bone marrow samples or in immunohistochemistry using cytospots or frozen tissue. Flow cytometry: please see "Protocols" below. Labelled reagent is effectively formulated for direct immunofluorescent staining. |
| Reactivities | Human |
| Host | Mouse |
| Isotype | IgG1 |
| Clonality | Monoclonal |
| Specificity | Clone NaM172-2B5 recognizes the human CD59 antigen. Other species not tested. |
| Formulation | 0.01 M sodium phosphate, 0.15 M NaCl, pH 7.3, 0.2% BSA, 0.09% sodium azide Label: FITC State: Liquid purified Ig fraction Label: Cat. No. Label EX-max (nm) / EM-max (nm): AM39024FC-N FITC 488 / 519 AM39024RP-N 488, 532 / 578 |
| Purification | Affinity chromatography |
| Conjugation | FITC |
| Storage | Store the antibody undiluted at 2-8°C. Fluorochrome labelled product is photosensitive and should be protected from light. |
| Stability | Shelf life: one year from despatch. |
| Gene Name | Homo sapiens CD59 molecule (CD59), transcript variant 2 |
| Database Link | |
| Background | CD59 can be found in body fluids including blood plasma, saliva, amniotic fluid, seminal fluid, and urine. It is expressed as a 18-25 kD glycoprotein (in lymphocytes)anchored in the membrane by GPI tail. Since CD59 is well known membrane-associated complement regulator protein, like CD55, and present on all blood cells, anti-CD55 and anti-CD59 appear to be the most effective Mabs to detect very minor negative cell subsets (less than 1% on erythrocytes or less than 5% on PMN leukocytes. |
| Synonyms | MAC-inhibitory protein, Protectin, MEM43 antigen, MIC11, MIN1, MIN2, MIN3, MSK21, MACIF, MAC-IP, MIRL, HRF20, HRF-20 |
| Note | 1. Conjugates with brighter fluorochromes, like PE and APC, will have a greater separation than those with dyes like FITC. When populations overlap, the percentage of positive cells using a selected marker can be affected by the choice of fluorescent label. 2. Use of monoclonal antibodies in patient treatment can interfere with antigen target recognition by this reagent. This should be taken into account when samples are analyzed from patients treated in this fashion. 3. Reagent data performance is based on EDTA-treated blood. Reagent performance can be affected by the use of other anticoagulants. Protocol: General flow cytometry method for use with labelled (FITC, R-PE, APC, PerCP or PerCP-Cy5.5) monoclonal antibodies 1. Add 100 µl of EDTA-treated blood (i.e. approx. 10e6 leukocytes) to a 5 ml reagent tube. The content of one tube is sufficient to perform one test. 2. Add to each tube 10 µl of labelled monoclonal antibody. (Appropriate mouse Ig isotype control samples should always be included in any labelling study). Vortex the tube to ensure thorough mixing of antibody and cells. 3. Incubate the tube for 15 minutes at room temperature in the dark. 4. Add 100 µl of a lyse reagent. 5. Incubate for 10 minutes at room temperature in the dark. 6. Add 2 ml of demineralized water and incubate for 10 minutes in the dark. 7. Centrifuge the labeled cell suspension for 2 minutes at 1000 x g. 8. Remove the supernatant and resuspend the cells in 200 µl of PBS. 9. Analyze by flow cytometry within four hours (alternatively, the cells may be fixed by 0.05% of formaline in buffered saline for analysis the next day. Some antigens are readily destroyed upon fixation and this should be taken into account when using this alternative). Flow cytometry method for use with dual and triple combinations 1. Add 100 µl of EDTA-treated blood (i.e. approx. 10e6 leukocytes) to a 5 ml reagent tube. The content of one tube is sufficient to perform one test. For combinations with anti-kappa and/or anti-lambda Ig see Application note below. 2. Add to each tube 20 µl of labelled monoclonal antibody combination. (Appropriate mouse Ig isotype control samples should always be included in any labelling study). 3. Vortex the tube to ensure thorough mixing of antibody and cells. 4. Incubate the tube for 15 minutes at room temperature in the dark. 5. Add 100 µl of a lyse reagent and mix immediately. 6. Incubate for 10 minutes at room temperature in the dark. 7. Add 2 ml of demineralized water and incubate for 10 minutes in the dark. 8. Centrifuge the labelled cell suspension for 2 minutes at 1000 x g. 9. Remove the supernatant and resuspend the cells in 200 µl of PBS. 10. Analyze by flow cytometry within four hours (alternatively, the cells may be fixed by 0.05% of formaline in buffered saline for analysis the next day. Some antigens are readily destroyed upon fixation and this should be taken into account when using this alternative). Application note for anti-kappa and/or anti-lambda Ig combinations Add 2 ml of PBS containing 0.001% (v/v) Heparin (prewarmed to 37°C) to the cell suspension Vortex, centrifuge (2 min at 300x g) and discard the supernatant. Repeat this step twice. Resuspend the pelleted blood cells in 100 µl PBS, pH 7.2, containing 0.001% (v/v) Heparin. Procedure for the diagnosis of Paroxysmal Nocturnal Hemoglobinuria (PNH) Erythrocytes -A- Preparation of Red Blood Cell Suspension 1. Use 10 ml of Heparin or EDTA whole blood and centrifuge 10 min. 600g (soft start/stop). 2. Collect the platelet rich plasma (PRP) and the buffy coat for further analysis of leukocytes and platelets, respectively. 3. Wash the pellet of erythrocytes three times with 2 ml of PBS for 2 min. 1000g. 4. Resuspend 1 volume of packed erythrocytes in 9 volumes of PBS. 5. Use a hemocytometer or automatic cell counter to calculate the total number of RBCs per ml blood collected in Heparin or EDTA treated tubes. 6. Dilute the counted RBCs with PBS to a final concentration of 50x10e6 cells/ml. -B- Immunofluorescent Staining 7. Determine the needed amount of tubes (negative control (= isotype control), positive control (= e.g. antiglycophorin A+B), CD55 and CD59 single or dual experiments). 8. Add 100 µl of RBCs to each tube (5x10e6 cells). 9. Add 10 µl of the singles (CD55, CD59) or 20 µl of the dual. 10. Incubate for 30 min. at room temperature. Avoid direct light. 11. Wash twice in 3 ml PBS and centrifuge for 2 min. 1000g. 12. Resuspend the cells in PBS (200-500 µl). -C- Flow Cytometry Data Acquisition 13. List mode files of 20.000 events should be collected for log FSC, log SSC and log fluorescence signals. Leukocytes -A- Preparation of Leukocyte Cell Suspension 1. Use 10 ml of Heparin or EDTA whole blood and centrifuge 10 min. 600g (soft start/stop). 2. Collect the platelet rich plasma (PRP) for further analysis of platelets. 3. Collect the buffy coat and add 10 ml of lysis buffer. 4. Incubate 5 min. at room temperature (maximum 10 min.). 5. Centrifuge 5 min. 400g to remove the lysis buffer. 6. Wash the pellet of leukocytes twice with 10 ml of PBS for 5 min. 400g. 7. Resuspend the pellet of leukocytes in 1 ml of PBS. 8. Use a hemocytometer or automatic cell counter to calculate the total number of leukocytes per ml blood collected in Heparin or EDTA treated tubes. 9. Dilute the counted leukocytes with PBS to a final concentration of 20x10e6 cells/ml. -B- Immuno-fluorescent Staining 10. Determine the needed amount of tubes (negative control (= isotype control), positive control (= e.g. anti-HLA class I), CD55 and CD59 single or dual experiments). 11. Add 100 µl of leukocytes to each tube (2x10e6 cells). 12. Add 10 µl of the singles (CD55, CD59) or 20 µl of the dual. 13. Incubate for 30 min. at room temperature. Avoid direct light. 14. Wash twice in 3 ml PBS and centrifuge for 4 min. 400g. 15. Resuspend the cells in PBS (200 – 500 µl). -C- Flow Cytometry Data Acquisition 16. Analyze at least 20.000 cells with the flow cytometer. Use gates based on morphological parameters in order to eliminate cell debris and electronic background and to separate lymphocytes, monocytes and granulocytes. Platelets Prepare PBS-EDTA 5 mM pH 7.4 (50 - 75 ml per patient). For best results 0,45 µm filtered PBS-EDTA 5 mM should be used. The PBS-EDTA 5 mM should be fresh (to be used during the running week) and must be filtrated before each experiment -A- Preparation of Platelet Cell Suspension 1. Use 10 ml of Heparin or EDTA whole blood and centrifuge 10 min. 600g (soft start/stop). 2. Collect the platelet rich plasma (PRP) and dilute in PBS-EDTA 5 mM, to a volume of 10 ml. 3. Centrifuge, 5 min. 2000g. 4. Discard supernatant and resuspend the pellet in 1 ml PBS-EDTA 5 mM. 5. Use a hemocytometer or automatic cell counter to calculate the total number of Platelets per ml blood collected in Heparin or EDTA treated tubes. 6. Dilute the counted platelets with PBS-EDTA 5 mM to a final concentration of 10x10e6 cells/ml. -B- Immuno-fluorescent Staining 7. Determine the needed amount of tubes (negative control (isotype control), positive control (CD61), CD55 and CD59 single or dual experiments). 8. Add 100 µl of leukocytes to each tube (2x10e6 cells). 9. Add 10 µl of the singles (CD55/CD59) or 20 µl of the dual. 10. Incubate for 30 min. at room temperature. Avoid direct light. 11. Wash twice in 3 ml PBS-EDTA 5 mM and centrifuge for 5 min. 2000g. 12. Resuspend the cells in PBS-EDTA 5 mM (200 – 500 µl). -C- Flow Cytometry Data Acquisition 13. For FACS analysis, use a gate based on morphological parameters in order to eliminate cell debris and electronic background List mode files of 20.000 events should be collected for log FSC, log SSC and log fluorescence signals. |
| Reference Data | |
| Protein Families | Druggable Genome |
| Protein Pathways | Complement and coagulation cascades, Hematopoietic cell lineage |
Documents
| Product Manuals |
| FAQs |
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China