Alpha B Crystallin (CRYAB) Rabbit Polyclonal Antibody
Other products for "CRYAB"
Specifications
| Product Data | |
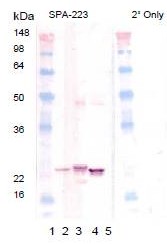
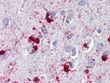
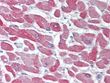
| Applications | IHC, WB |
| Recommended Dilution | Immunohistochemistry on Paraffin Sections: 10 µg/ml. Western Blot: 1/1000. |
| Reactivities | Bovine, Human, Mouse, Porcine, Rat |
| Host | Rabbit |
| Clonality | Polyclonal |
| Immunogen | CRYAB antibody was raised against synthetic peptide derived from sequence near the carboxy-terminus of human alphaB-Crystallin conjugated to KLH |
| Specificity | This antibody reacts to Alpha Crystallin B Chain (CRYAB). |
| Formulation | PBS containing 0.09% Sodium Azide as preservative and 50% glycerol State: Purified State: Liquid purified Ig fraction |
| Concentration | lot specific |
| Purification | Protein A Chromatography |
| Storage | Store undiluted at 2-8°C for one month or (in aliquots) at -20°C for longer. Avoid repeated freezing and thawing. |
| Stability | Shelf life: one year from despatch. |
| Gene Name | Homo sapiens crystallin alpha B (CRYAB), transcript variant 1 |
| Database Link | |
| Background | Alpha-crystallins composed of ~20 kDa alphaA and alphaB subunits function as major water-soluble proteins accounting for almost 50% of total protein in the mammalian transparent eye lens, also existing in a variety of other tissues1. Crystallin families beta and gamma share homology with each other but not the alpha-crystallin family or the small heat shock protein (sHsp) family. sHsps including the alpha- crystallin proteins are induced by heat and other stress insults in a variety of organisms. The alpha-crystallins possess structural and functional similarities and share sequence homology with Hsp25/273. Most sHsps exhibit four common structural and functional features: monomeric molecular weight between 12 and 43kDa; the formation of large oligomeric complexes especially for alphaA-crystallin, alphaB-crystallin and Hsp25/27; a moderately conserved alpha-crystallin domain in the central region of the protein; and molecular chaperone activity. The alpha-crystallin domain bounded by variable N-terminal and C-terminal extensions contains approximately 80 residues and participates in oligomer assembly. Oligomers, potentially 800kDa or more, exhibit dynamic subunit exchanges and organizational plasticity, which may promote functional diversity. Phosphorylation of serine residues specifically for Hsp27 occurs in response to stress during development, typically decreasing oligomer size. Chaperone activity requires oligomerization (which, in turn, modulates the chaperone activity) and is confined to binding unfolded intermediates to prevent irreversible aggregation, even though productive release and refolding of denatured proteins requires close cooperation with other chaperones. Other proposed functions include a role in membrane stabilization and modulation of intermediate filament organization during physiological stress and neurodegenerative disease. |
| Synonyms | Alpha(B)-crystallin, CRYAB, Heat shock protein beta-5, Renal carcinoma antigen NY-REN-27, HspB5 |
| Reference Data | |
Documents
| Product Manuals |
| FAQs |
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China