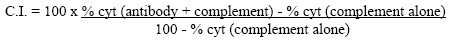CD45 / LCA (CD45R) Mouse Monoclonal Antibody [Clone ID: S-450-15.2]
Specifications
| Product Data | |
| Clone Name | S-450-15.2 |
| Applications | Assay, CT, FN, WB |
| Reactivities | Mouse |
| Host | Mouse |
| Isotype | IgG2a |
| Clonality | Monoclonal |
| Immunogen | Recipient: SJL/J Donor: BIO.S (thymocytes, spleen, and lymph node cells) Fusion Partner: P3-NSI/1-Ag (NS-1) |
| Specificity | This monoclonal antibody detects a 180 kDa isoform in thymus, spleen, and lymph node tissue as determined by SDS-PAGE. An additional antigen of 200 kDa was precipitated from splenic tissue and a very small amount of protein of approximately 180 kDa was precipitated from bone marrow tissue (5). This antibody in the presence of complement can be used to deplete/identify a cell population of Ly 5.2 bearing cells or used in the absence of complement to block natural killer activity. |
| Formulation | State: Ascites State: Lyophilized Ascites |
| Reconstitution Method | Restore with 0.5 ml of distilled water. |
| Background | Ly 5 is a major cell surface component of T and B lymphocytes and most hematopoietic cells including promyelocytes, metamyelocytes, macrophages, eosinophils and neutrophils but is absent from mature erythroid cells (1). An interesting feature of the Ly 5 system is that members of this family of glycoproteins range in size from approximately 180 kDa to 240 kDa and differ in both protein sequence and carbohydrate content (2). Ly 5 alloantigens may be present in one of several molecular isoforms, and it has been postulated that each defines stages and lineages of hematopoietic differentiation (2). B lymphocytes express a 220 kDa form (B220) (3), while T lymphocytes indicate a more complicated pattern of expression with at least four forms identified by SDS-PAGE (2). Different patterns of expression have been identified between T lymphocyte subsets, indicating that expression of individual members is controlled in a cell-type specific fashion. Individual T cells may also express more than one member of the Ly 5 family and during activation, expression of the various forms may change (4). Additionally, there appears to be further Ly 5 modifications that occur as mature thymocytes migrate to peripheral lymphoid organs (4). Thus, the Ly 5 antigen exhibits inter and intratissue polymorphism, and it becomes clear that a programmed sequence of Ly 5 expression occurs during T cell differentiation and activation and that the forms expressed by lymphocyte populations are dynamic. The Ly 5.2 antigen has also been defined as a major component of natural killer cells and it has been further suggested that the Ly 5 (or modified Ly 5) may be part of or close to a receptor on natural killer cells, for their target (6,7). |
| Synonyms | PTPRC, Leukocyte common antigen, L-CA, T200 |
| Note | This reagent is not sold as sterile, but can be sterilized by filtration if necessary. To minimize loss of volume during filtration, dilute to the final working concentration in the appropriate medium before filtration and filter through a 0.22 µ Millipore filter (or equivalent). Protocol: RECOMMENDED METHOD FOR DEPLETING A CELL POPULATION OF LY 5.2 POSITIVE LYMPHOCYTES: 1. Prepare a cell suspension from the appropriate tissue in Cytotoxicity Medium1 or equivalent. Remove red cells and dead cells (where necessary) by purification of viable lymphocytes on Lympholyte®-M density cell separation medium. After washing, adjust the cell concentration to 1x10e7 cells per ml in Cytotoxicity Medium. 2. Add the antibody to a final concentration of 1:20 and mix. Alternately, pellet the cells and resuspend in antibody diluted 1:20 in Cytotoxicity Medium. 3. Incubate for 60 minutes at 4°C. 4. Centrifuge to pellet the cells and discard the supernatant. 5. Resuspend to the original volume in Low-Tox®-M Rabbit Complement3, diluted to the appropriate concentration in Cytotoxicity Medium. (Recommended concentration included with each batch of Low-Tox®-M Rabbit Complement.) 6. Incubate for 60 minutes at 37°C. 7. Monitor for percent cytotoxicity at this stage before further processing. For this purpose, remove a small sample from each tube, dilute 1:10 with medium, and add 1/10 volume of 1% Trypan Blue. After 3-5 minutes, score live versus dead cells in a hemocytometer. 8. For functional studies, remove the dead cells from the treated groups before further processing, particularly if the treated cells are to be cultured. This can be done by layering the treated cell suspensions over an equal volume of Lympholyte®-M cell separation medium and centrifuging at room temperature as per the instructions provided. Live cells will form a layer at the interface, while the dead cells pellet. The interface can then be collected and washed in Cytotoxicity Medium before being resuspended in the appropriate medium for further processing. Alternately, the cells can be washed and resuspended in the appropriate medium for further processing immediately after Step # 6, provided that the dead cells will not interfere with subsequent assays. RECOMMENDED METHOD FOR DETERMINING PERCENT OF LY 5.2 POSITIVE CELLS IN A POPULATION: 1. Prepare a cell suspension from the appropriate tissue in Cytotoxicity Medium1 or equivalent. Remove red cells and dead cells (where necessary) by purification of viable lymphocytes on Lympholyte®-M density cell separation medium. After washing, adjust the cell concentration to 1x10e6 cells per ml in Cytotoxicity Medium. 2. Add the antibody to a final concentration of 1:100 and mix. 3. Incubate for 60 minutes at 4°C. 4. Centrifuge to pellet the cells and discard the supernatant. 5. Resuspend to the original volume in Low-Tox®-M Rabbit Complement3, diluted to the appropriate concentration in Cytotoxicity Medium. (Recommended concentration included with each batch of Low-Tox®-M Rabbit Complement.) 6. Incubate for 60 minutes at 37°C. 7. Place on ice. 8. Add Trypan Blue, 10% by volume of 1% Trypan Blue (w/v) added 3 to 5 minutes before scoring works well. Score live versus dead cells in a hemacytometer. 9. Cytotoxic Index see Pictures NOTES: 1. Cytotoxicity Medium is RPMI-1640 with 25 mM Hepes buffer and 0.3% bovine serum albumin (BSA). BSA is substituted for the conventionally used fetal calf serum (FCS) because we have found that many batches of FCS contain complement dependent cytotoxins to mouse lymphocytes, thus increasing the background killing in the presence of complement. We recommend that cells not be exposed to FCS prior to or during exposure to antibody and complement. Some batches of BSA also contain complement dependent cytotoxins, resulting in the same problem. We screen for batches of BSA giving low background in the presence of complement and use the selected BSA for preparing Cytotoxicity Medium. 2. Lympholyte®-M cell separation medium is a density separation medium designed specifically for the isolation of viable mouse lymphocytes. This separation medium provides a high and non-selective recovery of viable mouse lymphocytes, removing red cells and dead cells. The density of this medium is 1.087-1.088. Isolation of mouse lymphocytes on cell separation medium of density 1.077 will result in high and selective loss of lymphocytes and should be avoided. 3. Rabbit serum provides the most potent source of complement for use with antibodies to mouse cell surface antigens. However, rabbit serum itself is very toxic to mouse lymphocytes. Low-Tox®-M Rabbit Complement is absorbed to remove toxicity to mouse lymphocytes, while maintaining its high complement activity. When used in conjunction with Cytotoxicity Medium, this reagent provides a highly potent source of complement with minimal background toxicity. 4. Ly 5, formerly Ly 4. The allele and specificity designations originally described are reversed, i.e. C57BL/6 now carries the Ly 5b allele and the Ly 5.2 specificity, and SJL carries the Ly 5a allele and the Ly 5.1 specificity, (8). RESULTS - STRAIN DISTRIBUTION: Procedure: As above Antibody Concentration: 1:40 Strains Tested: C3H/He, CBA/J, BALB/c, C57BL/6, SJL/J, AKR/J,B6 Lyt 2.1 3.1 Cells Killed by Treatment: C3H/He, CBA/J, BALB/c, C57BL/6, AKR/J, B6 Lyt 2.1 3.1 Cells Not Killed by Treatment: SJL/J RESULTS - TISSUE DISTRIBUTION: Procedure: As above Antibody Concentration: 1:40 Strain: C3H/He CELL SOURCE C.I. Thymus: 86 Spleen: 15 Lymph Node: 20 Bone Marrow: 1 FUNCTIONAL TESTING: Method: Cells were treated as described in Recommended Method for Depleting a Cell Population of Ly 5.2 Positive Lymphocytes. Treated cells and controls were tested in vitro for a) the ability to generate plaque-forming cells (PFC) using a modified Jerne hemolytic plaque assay and b) the ability to generate cytotoxic T effector cells using a cytotoxic lymphocyte reaction (CTL) assay. A51Cr-release natural killer cell lysis assay was performed using BALB/c effector cells and the NK sensitive tumour cell line, YAC-1, as target cells. Results: Cell Source: Spleen Donors: BALB/c, C3H/He Cell Concentration: 1x10e7 cells/ml Antibody Concentration: 1:20 Complement: Low-Tox®-M Rabbit Complement Complement Concentration: 1:10 Treatment of BALB/c and C3H/He cells with anti-Ly 5.2 plus complement had no effect on the number of plaque-forming cells. Cytotoxic T cell function as assessed by CTL assay was found to be markedly inhibited in samples treated before or after sensitization. Results of the NK assay indicated that there was significant inhibition of NK cell lysis by anti-Ly 5.2 observed both with and without the addition of a complement source. These results are consistent with the depletion and/or inactivation of Ly 5.2 bearing cells. |
| Reference Data | |
Documents
| Product Manuals |
| FAQs |
| SDS |
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China