MHC Class I H-2 Kb / H-2 Db Mouse Monoclonal Antibody [Clone ID: 5041.16.1]
CAT#: CL058A
MHC Class I H-2 Kb / H-2 Db mouse monoclonal antibody, clone 5041.16.1, Azide Free
Specifications
| Product Data | |
| Clone Name | 5041.16.1 |
| Applications | Assay, CT |
| Recommended Dilution | Cytotoxicity assays. Functional testing. (For details please see "Protocols" below). |
| Reactivities | Mouse |
| Host | Mouse |
| Isotype | IgG2a |
| Clonality | Monoclonal |
| Immunogen | Recipient: CBA/J. Donor: bm12 (thymus, lymph node and spleen cells). Fusion Partner: NS-1. |
| Specificity | This antibody is specific for cells expressing the H-2K antigen coded for by the b haplotype and for cells expressing the H-2D antigen coded for by the b haplotype. Strain Distribution: H-2Kb and/or H-2Db positive strains. |
| Formulation | PBS, without preservatives State: Azide Free State: Liquid purified Ig fraction |
| Concentration | 1.0 mg/ml |
| Purification | Protein G Chromatography |
| Synonyms | HLA Class I |
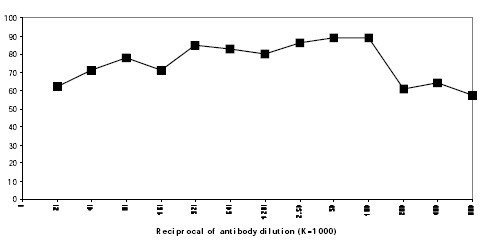
| Note | Protocol: RECOMMENDED METHOD FOR DEPLETING A CELL POPULATION OF H-2Kb AND/OR H-2Db BEARING LYMPHOCYTES: 1. Prepare a cell suspension from the appropriate tissue in a cytotoxicity medium (1) or equivalent. Remove red cells and dead cells (where necessary) by purification of viable lymphocytes on a density cell separation medium (2). After washing, adjust the cell concentration to 1x10e7 cells per ml in cytotoxicity medium. 2. Add the antibody to a final concentration of 1:100 and mix. Alternatively, pellet the cells and resuspend in antibody diluted 1:100 in cytotoxicity medium. 3. Incubate for 60 minutes at 4°C. 4. Centrifuge to pellet the cells and discard the supernatant 5. Resuspend to the original volume in a low-toxicity rabbit complement (3), diluted to the appropriate concentration in cytotoxicity medium. 6. Incubate for 60 minutes at 37°C. 7. Monitor for percent cytotoxicity at this stage, before further processing. For this purpose remove a small sample from each tube, dilute 1:10 with medium, and add 1/10 volume of 1% Trypan Blue. After 3-5 minutes, score live versus dead cells in a haemacytometer. 8. For functional studies, remove the dead cells from the treated groups before further processing, particularly if the treated cells are to be cultured. This can be done by layering the treated cell suspensions over an equal volume of cell suspension separation medium and centrifuging at room temperature as per the instructions provided. Live cells will form a layer at the interface, while the dead cells pellet. The interface can then be collected and washed in cytotoxicity medium before being resuspended in the appropriate medium for further processing. Alternately, the cells can be washed and resuspended in the appropriate medium for further processing immediately after Step 6., provided that the dead cells will not interfere with subsequent assays. RECOMMENDED METHOD FOR DETERMINING PERCENT OF H-2Kb AND/OR H-2Db BEARING CELLS IN A POPULATION: 1. Prepare a cell suspension from the appropriate tissue in cytotoxicity medium or equivalent. Remove red cells and dead cells (where necessary) by purification of viable lymphocytes on density cell separation medium. After washing, adjust the cell concentration to 1x10e6 cells per ml in cytotoxicity medium. 2. Add the antibody to a final concentration of 1:1000 and mix. 3. Incubate for 60 minutes at 4°C. 4. Centrifuge to pellet the cells and discard the supernatant. 5. Resuspend to the original volume in Rabbit Complement diluted to the appropriate concentration in cytotoxicity edium. 6. Incubate for 60 minutes at 37°C. 7. Place on ice. 8. Add Trypan Blue, 10% by volume of 1% Trypan Blue (w/v) added 3-5 minutes before scoring works well. Score live versus dead cells in a haemacytometer. Cytotoxic Index (C.I.) can be calculated as follows: C.I. = 100 x %cyt. (antibody + complement) - % cyt. (complement alone) / 100 - % cyt. (complement alone) Media: (1) The cytotoxicity medium used is RPMI-1640 with 25mM Hepes buffer and 0.3% bovine serum albumin (BSA). BSA is substituted for the conventionally used fetal calf serum (FCS) because we have found that many batches of FCS contain complement dependent cytotoxins to mouse lymphocytes, thus increasing the background killing in the presence of complement. We recommend that cells not be exposed to FCS prior to or during exposure to antibody and complement. Some batches of BSA also contain complement dependent cytotoxins, resulting in the same problem. We screen for batches of BSA giving low background in the presence of complement and use the selected BSA for preparing the cytotoxicity medium. (2) Cell separation medium is density separation medium designed specifically for the isolation of viable mouse lymphocytes. This separation medium provides a high and non-selective recovery of viable mouse lymphocytes, removing red cells and dead cells. The density of this medium is 1.087-1.088. Isolation of mouse lymphocytes on cell separation medium of density 1.077 will result in high and selective loss of lymphocytes and should be avoided. (3) Rabbit serum provides the most potent source of complement for use with antibodies to mouse cell surface antigens. However, rabbit serum itself is very toxic to mouse lymphocytes. Use a low-toxicity rabbit complement which is absorbed to remove toxicity to mouse lymphocytes, while maintaining its high complement activity. RESULTS: Antibody Titration: Cell Source: Splenocytes Donors: C57BL/6 Cell concentration: 1x106 cells per ml Complement: Low toxicity rabbit complement (complement concentration: 1:12) Procedure: Two stage cytotoxicity as described in 'Recommended Method for Determining Percent of H-2Kb or H-2Db bearing cells in a Population'. C.I. = 100 x %cyt. antibody + complement) - % cyt. (complement alone) / 100 - % cyt. (complement alone) (see picture below) Strain Distribution: Procedure: as above Antibody concentration: Final concentration 1:100 Strains Tested: C3H/He, C57BL/6, CBA/J, AKR/J, BALB/c Cells Killed by Treatment: C57BL/6 Tissue Distribution: Procedure: as above Antibody concentration: 1:320 Strain: C57BL/6 Cell Source / Cytotoxic Index Spleen / 85 Thymus / 55 Lymph Node / 87 Bone Marrow / 71 FUNCTIONAL TESTING: Cell Source: Spleen Donors: C57BL/6, BALB/c Cell concentration: 1x10e7 cells/ml Antibody concentration: 1:100 Complement: Low toxicity rabbit complement (complement concentration: 1:10) Procedure: Cells were treated as described in 'Recommended Method for Depleting a Population of H-2Kb or H-2Db Bearing Lymphocytes' . Treated cells and controls were tested for a) the ability to generate plaque-forming cells (PFC) using a modified Jerne haemolytic plaque assay and b) the ability to generate cytotoxic T effector cells using a cytotoxic lymphocyte reaction (CTL) assay. Cells were treated both before and after sensitization in the CTL assay. In vitro immunizations were used in all experiments. RESULTS: Treatment of C57BL/6 splenocytes with anti-H-2KbDb plus complement essentially eliminates plaque-forming cells and cytotoxic T cells, as assessed by Jerne haemolytic plaque assay and CTL assay, respectively. Treatment of BALB/c splenocytes had no effect. These results are consistent with the depletion of H- 2KbDb bearing cells. |
| Reference Data | |
Documents
| Product Manuals |
| FAQs |
| SDS |
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China