H2-Q7 Mouse Monoclonal Antibody [Clone ID: 5035-50.1]
Specifications
| Product Data | |
| Clone Name | 5035-50.1 |
| Applications | Assay, CT, FC |
| Recommended Dilution | Cytotoxicity Assay. Flow Cytometry. |
| Reactivities | Mouse |
| Host | Mouse |
| Isotype | IgM |
| Clonality | Monoclonal |
| Immunogen | Recipient: BALB/c By Donor: B6.Ly1a Fusion Partner: P3-NSI/1-Ag4(NS-1) |
| Specificity | This antibody reacts to MHC Class I Qa.m7. This monoclonal Qa.m7 antibody detects the Qa.m7 antigenic determinant on molecule coded by H-2 linked by mapping within the Qa region. It is possible that this specificity represents a discrete epitope on previously described Loci (Qa-2) rather than a product of new Qa loci, which may code for antigenically distinct molecules. In fact, this Qa.m7 antibody defines a Qa antigen which appears to be molecularly similar or identical to antigen defined by antibodies to other Qa specification (i.e. Qa.m2, Qa.m8, Qa.m9). Biochemical studies have inidcated that Qa.m7 is found on a molecule consisting of a 39,000d heavy chain and a 12,000d light chain wgich is B2, Microglobulin. Qa.m7 is distinguished from other Qa specificities on the basis of unique strain and distribution pattern obtained. It has been identified on >90% of spleen and lymph node cells and ~67% of bone marrow cells but is absent on lymphocytes. This antibody, in conjunction with others (i.e. anti-Qa.m8 antibody) could prove useful in defining subsets of functional cells. Examples: Qa.m7: >90% T cells, 70% B cells. Qa.m8: 70% T cells, 0% B cells. As each of these antigens are present on subsets of T cells (Qa.m8), B cells (Qa.m7) and bone marrow cells (Qa.m7 and Qa.m8), it may be used to define subsets of functional cells. |
| Formulation | State: Ascites State: Lyophilized Ascites |
| Reconstitution Method | Restore with 0.5 ml distilled water. |
| Database Link | |
| Background | The Qa region, so named because of the closely linked Qa 1-9 loci, is situated on murine chromosome 17 between the H-2D and T1a loci. These Qa loci code for cell surface alloantigens expressed on some lymphoid and myeloid cells. Their expression appears to be controlled by a gene or genes closely linked to the H-2D locus and they have been identified as being structurally related to the murine H-2 antigens. Although no functional role for Qa antigens has been determined, the restricted and differential distribution of Qa determinants on hemopoietic and lymphoid cells raises the possibility that they may not be simply differentiation markers but may play a role in hematopoetic differentiation. |
| Synonyms | H2-Q7 |
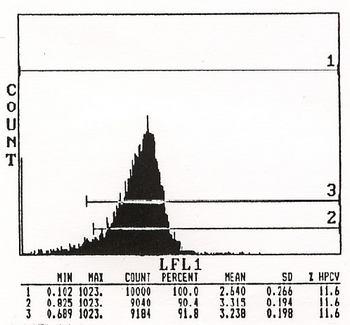
| Note | The reagent is not sold as sterile, but can be sterilized by filtration if necessary. To minimize loss of volume during filtration, dilute to the final working concentration in the appropriate medium before filtration and filter through a 0.45 µ millipore filter (or equivalent). Protocol: RECOMMENDED METHOD FOR DEPLETING A CELL POPULATION OF Qa.m7 POSITIVE LYMPHOCYTES 1. Prepare a cell suspension from the appropriate tissue in Cytotoxicity Medium or equivalent. Remove red cells and dead cells (where necessary) by purification of viable lymphocytes on Lympholyte®-M density cell separation medium. After washing adjust the cell concentration to 1 x 10e7 cells per ml in Cytotoxicity Medium. 2. Add the antibody to a final concentration 1/20 and mix. Alternately, pellet the cells and resuspend in antibody diluted 1/20 in Cytotoxicity Medium. 3. Incubate for 60 minutes at 4°C. 4. Centrifuge to pellet the cells and discard the supernatant. 5. Resuspend to the original volume in Low-Tox®-M Rabbit Complement3, diluted to the appropriate concentration in Cytotoxicity Medium. (Recommended concentration included with each batch of Low-Tox®-M Rabbit Complement.) 6. Incubate for 60 minutes at 37°C. 7. Monitor for percent cytotoxicity at this stage, before further processing. For this purpose, remove a small sample from each tube, dilute 1/10 with medium, and add 1/10 volume of 1% trypan blue. After 3-5 minutes, score live vs. dead cells in a hemacytometer. 8. For functional studies, remove the dead cells from the treated groups before further processing, particularly if the treated cells are to be cultured. This can be done by layering the treated cell suspensions over an equal volume of Lympholyte®-M cell separation medium and centrifuging at room temperature as per the instruction provided. Live cells will form a layer at the interface, while the dead cells pellet. The interface can then be collected and washed in Cytotoxicity Medium before being resuspended in the appropriate medium for further processing. Alternately, the cells can be washed and resuspended in the appropriate medium for further processing immediately after Step#6, provided that the dead cells will not interfere with subsequent assays. RECOMMENDED METHOD FOR DETERMINING PERCENT OF Qa.m7 POSITIVE CELLS IN A POPULATION 1. Prepare a cell suspension from the appropriate tissue in Cytotoxicity Medium1 or equivalent. Remove red cells and dead cells (where necessary) by purification of viable lymphocytes on Lympholyte®-M density cell separation medium. After washing, adjust the cell concentration to 1 x 10e6 cells per ml in Cytotoxicity Medium. 2. Add the antibody to a final concentration of 1/40K and mix. 3. Incubate for 60 minutes at 4°C. 4. Centrifuge to pellet the cells and discard the supernatant. 5. Resuspend to the original volume in Low-Tox®-M Rabbit Complement3, diluted to the appropriate concentration in Cytotoxicity Medium. (Recommended concentration included with each batch of Low-Tox®-M Complement). 6. Incubate for 60 minutes at 37°C. 7. Place on ice. 8. Add trypan blue. 10% volume of 1% trypan blue (w/v) added 3-5 minutes before scoring works well. Score live vs. dead cells in a hemacytometer. Cytotoxic Index (C.I.) can be calculated as shown in FIGURE 1. Notes: 1. Cytotoxicity Medium® is RPMI-1640 with 25mM Hepes buffer and 0.3% bovine serum albumin (BSA). BSA is substituted for the conventionally used fetal calf serum (FCS) because we have found that many batches of FCS contain complement dependent cytotoxins to mouse lymphocytes, thus increasing the background killing in the presence of complement. We recommend that cells not be exposed to FCS prior to or during exposure to antibody and complement. Some batches of BSA also contain complement dependent cytotoxins, resulting in the same problem. We screen for batches of BSA giving low background in the presence of complement and use the selected BSA for preparing Cytotoxicity Medium®. 2. Lympholyte®-M cell separation medium is a density separation medium designed specifically for the isolation of viable mouse lymphocytes. This separation medium provides a high and non-selective recovery of viable mouse lymphocytes, removing red cells and dead cells. The density of this medium is 1.087-1.088. Isolation of mouse lymphocytes on cell separation medium of density 1.077 will result in high and selective loss of lymphocytes and should be avoided. 3. Rabbit serum provides the most potent source of complement for use with antibodies to mouse cell surface antigens. However, rabbit serum itself is very toxic to mouse lymphocytes. Low-Tox®-M Rabbit Complement is absorbed to remove toxicity to mouse lymphocytes, while maintaining its high complement activity. When used in conjunction with Cytotoxicity Medium, this reagent provides a highly potent source of complement with minimal background toxicity. Antibody Titration: See FIGURE 2. Cell Source: spleen Donor: C57BL/6 Cell Concentration: 1.1x10e6 cells/ml Complement: Low-Tox®-M Rabbit Complement Complement Concentration: 1/10 Procedure: Two stage cytotoxicity as described in Recommended Method for Determining Percent of Qa.m7 Positive Lymphocytes. Strain Distribution: Procedure: As above Antibody Concentration: 1/40 Strains tested: C57BL/6, BALB/c, C3H/he, CBA, AKR, DBA/2 Cells killed by treatment: BALB/c, DBA/2 Cells not killed by treatment: BALB/c, C3H/He, CBA, AKR Tissue Distribution: Procedure: as above Antibody Concentration: 1/40,000 Strain: C57BL/6 Cell Source C.I. Thymus: 8 Spleen: 74 Lymph Node: 82 Bone Marrow: 37 FLOW CYTOMETRY ANALYSIS OF SPLENIC T CELLS: See FIGURE 4. DONOR: C57BL/6 CELL CONCENTRATION: 1 x 10e6 cells per test. ANTIBODY CONCENTRATION: 1/500 CELL SOURCE: Splenic T Cells RECOMMENDED METHOD FOR LABELLING Qa.m7 POSITIVE CELLS 1. Prepare cell suspension in media A. For cell preparations, deplete the red blood cell population with Lympholyte®-M cell separation medium. Wash 2 times. Resuspend cells to 1 x 10e6 cells in approximately 50 µl media A in a microcentrifuge tube (ie. 50 µl of cells resuspended to 2x10e7 cells / ml). The contents of one tube represent one test. 2. To each tube add 50 µl of a 1/250 dilution of this Ab (to make a final dilution of 1/500). 3. Vortex the tubes to ensure thorough mixing of antibody and cells. 4. Incubate the tubes for 30 minutes at 4°C. 5. Wash 2 times at 4°C. 6. Add 100 µl of secondary antibody (Goat anti-mouse IgG (H+L) FITC conjugate) at a 1/75 dilution. 7. Incubate tubes at 4°C for 30-60 minutes. 8. It is recommended that the tubes are protected from light since most fluorochromes are light sensitive. 9. Wash 2 times at 4°C in media B. 10. Resuspend the cell pellet in 50 µl ice cold media B. 11. Transfer to suitable tubes for flow cytometric analysis containing 15 µl of propidium iodide at 0.5 mg/ml in phosphate buffered saline - (this stains dead cells by intercalating in DNA). N.B. Appropriate control samples should always be included in any labelling studies. Media: A. Phosphate buffered saline (pH 7.2) + 0.5% normal serum of host species + sodium azide (100 µl of 2M sodium azide in 100 mls). B. Phosphate buffered saline (pH 7.2) + 0.5% bovine serum albumin + sodium azide (100 µl of 2M sodium azide in 100 mls). |
| Reference Data | |
Documents
| Product Manuals |
| FAQs |
| SDS |
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China