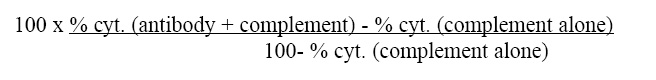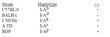MHC Class II I-Ap Mouse Monoclonal Antibody [Clone ID: 7-16.17]
Specifications
| Product Data | |
| Clone Name | 7-16.17 |
| Applications | Assay, CT, FC |
| Recommended Dilution | Cytotoxicity analysis. |
| Reactivities | Mouse |
| Host | Mouse |
| Isotype | IgG2a |
| Clonality | Monoclonal |
| Immunogen | Recipient: BALB/c Donor: B10.P Fusion Partner: Spleen from immunized recipient fused with myeloma SP2/0 |
| Specificity | This monoclonal antibody is a cytotoxic antibody which defines a new public I-A antigen. This antibody reacts with I-A antigen from the following I-A haplotypes: I-Ap,k,q,r,s.j,b. Using recombinant strains, reactivity against the b haplotype has been localized to the Ab subregion. This antibody can be used to quantitate or eliminate I-A bearing cells for precipitating I-A antigen. |
| Formulation | PBS State: Ig Fraction State: Lyophilized fraction of ascites |
| Reconstitution Method | Restore with 1.0 ml of distilled water. |
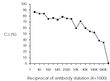
| Note | This reagent is not sold as sterile, but can be sterilized by filtration if necessary. To minimize loss of volume during filtration, dilute to the final working concentration in the appropriate medium before filtration. Protocol: RECOMMENDED METHOD FOR DEPLETING A CELL POPULATION OF I-A ANTIGEN BEARING CELLS: 1. Prepare a cell suspension from the appropriate tissue in Cytotoxicity Medium®1 or equivalent. Remove red cells and dead cells (where necessary) by purification of viable lymphocytes on Lympholyte-M®2 density cell separation medium. After washing, adjust the cell concentration to 1 x 10e7 cells per ml in Cytotoxicity Medium. 2. Add the antibody to a final concentration of 1:40 and mix. Alternatively, pellet the cells and resuspend in antibody diluted 1:40 in Cytotoxicity Medium. 3. Incubate for 60 minutes at 4°C. 4. Centrifuge to pellet the cells and discard the supernatant. 5. Resuspend to the original volume in Low-Tox-M® Rabbit Complement3, diluted to the appropriate concentration in Cytotoxicity Medium. (Recommended concentration included with each batch of Low-Tox-M® Rabbit Complement.) 6. Incubate for 60 minutes at 37°C. 7. Monitor for percent cytotoxicity at this stage, before further processing. For this purpose, remove a small sample from each tube, dilute 1:10 with medium, and add 1/10 volume of 1% trypan blue. After 3-5 minutes, score live versus dead cells in a hemacytometer. 8. For functional studies, remove the dead cells from the treated groups before further processing, particularly if the treated cells are to be cultured. This can be done by layering the cell suspension over a separation medium and centrifuging at room temperature as per the instructions provided. Live cells will form a layer at the interface , while the dead cells pellet. The interface can then be collected and washed in Cytotoxicity Medium before being resuspended in the appropriate medium for further processing. Alternatively, the cells can be washed and resuspended in the appropriate medium for further processing immediately after Step #6, provided that the dead cells will not interfere with subsequent assays. RECOMMENDED METHOD FOR DETERMINING PERCENT OF I-A ANTIGEN BEARING CELLS IN A POPULATION: 1. Prepare a cell suspension from the appropriate tissue in Cytotoxicity Medium®1 or equivalent. Remove red cells and dead cells (where necessary) by purification of viable lymphocytes on Lympholyte-M® density cell separation medium. After washing, adjust the cell concentration to 1 x 10e6 cells per ml in Cytotoxicity Medium. 2. Add the antibody to a final concentration of 1:80 and mix. 3. Incubate for 60 minutes at 4°C. 4. Centrifuge to pellet the cells and discard the supernatant. 5. Resuspend to the original volume in Low-Tox-M® Rabbit Complement3 diluted to the appropriate concentration in Cytotoxicty Medium. (Recommended concentration included with each batch of Low-Tox-M® Rabbit Complement). 6. Incubate for 60 minutes at 37°C. 7. Place on ice. 8. Add trypan blue. 10% by volume of 1% trypan blue (w/v) added 3-5 minutes before scoring works well. Score live vs dead cells in a hemacytometer. Cytotoxic index (C. I.) can be calculated as shown in FIGURE 1 NOTES: 1. Cytotoxicity Medium® is RPMI - 1640 with 25mM Hepes buffer and 0.3% bovine serum albumin (BSA). BSA is substituted for the conventionally used fetal calf serum (FCS) because we have found that many batches of FCS contain complement dependent cytotoxins to mouse lymphocytes, thus increasing the background killing in the presence of complement. We recommend that cells not be exposed to FCS prior to or during exposure to antibody and complement. Some batches of BSA also contain complement dependent cytotoxins, resulting in the same problem. We screen for batches of BSA giving low background in the presence of complement and use the selected BSA for preparing Cytotoxicity Medium ®. 2. Lympholyte-M® cell separation medium is density separation medium designed specifically for the isolation of viable mouse lymphocytes. This separation medium provides a high and non-selective recovery of viable mouse lymphocytes, removing red cells and dead cells. The density of this medium is 1.087 - 1.088. Isolation of mouse lymphocytes on cell separation medium of density 1.077 will result in high and selective loss of lymphocytes and should be avoided. 3. Rabbit serum provides the most potent source of complement for use with antibodies to mouse cell surface antigens. However, rabbit serum itself is very toxic to mouse lymphocytes. Low-Tox-M® Rabbit Complement is absorbed to remove toxicity to mouse lymphocytes, while maintaining its high complement activity. When used in conjunction with Cytotoxicity Medium®,this reagent provides a highly potent source of complement with minimal background toxicity. ANTIBODY TITRATION: CELL SOURCE: BDP (I-Ap) enriched splenic B cells CELL CONCENTRATION: 1 x 10e6 cells per ml COMPLEMENT: Low-Tox-M® Rabbit Complement COMPLEMENT CONCENTRATION: 1:12 see FIGURE 2 PROCEDURE: Two stage cytotoxicity as described in Recommended Method for Determining Percent of I-A Antigen Bearing Cells in a Population. STRAIN DISTRIBUTION: Procedure: as above Antibody Concentration: 1:40 Strains tested: see FIGURE 3 TISSUE DISTRIBUTION: Procedure: As above Antibody Concentration: 1:80 Strain: BDP (I-Ap) Cell Source - C.I. Spleen: 36 Thymus: 3 Lymph Node: 21 Enriched Splenic B-cells*: 86 *Enriched Splenic B-cells prepared by treatment of Lympholyte®-M isolated spleen lymphocytes with Monoclonal Anti-Thy-1.2 antibody plus complement, followed by removal of dead cells on Lympholyte®-M. FUNCTIONAL TESTING: Cell Source: Splenocytes Donors: BALB/c and C57BL/6 Cell Concentration: 1 x 10e7 cells/ml Antibody Concentration: 1:20 Complement: Low-Tox®-M Rabbit Complement Complement Concentration: 1:10 PROCEDURE: Cells were treated as described in Recommended Method for Depleting a Cell Population of I-A Antigen Bearing Lymphocytes. Treated cells and controls were tested for: a) the ability to generate plaque-forming cells (PFC) using a modified Jerne haemolytic plaque assay and b) the ability to generate cytotoxic T effector cells using a cytotoxic lymphocyte reaction (CTL) assay. Cells were treated both before and after sensitization in the CTL assay. In vitro immunizations were used in all experiments. RESULTS: Treatment of C57BL/6 splenocytes with anti-I-Ap plus complement resulted in a marked reduction in the number of plaque-forming cells. Partial inhibition of cytotoxic T cell function as assessed by CTL assay was also noted. No effect in either assay was observed when BALB/c cells were used. These results are consistent with the removal of I-Ap bearing cells and their related activities. |
| Reference Data | |
Documents
| Product Manuals |
| FAQs |
| SDS |
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China