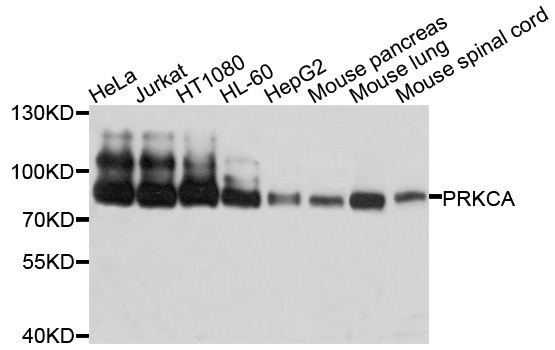
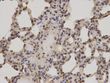
PKC alpha (PRKCA) Rabbit Polyclonal Antibody
Frequently bought together (2)
Transient overexpression lysate of protein kinase C, alpha (PRKCA)
USD 605.00
Other products for "PRKCA"
Specifications
| Product Data | |
| Applications | IHC, WB |
| Recommended Dilution | WB 1:500 - 1:2000;IHC 1:50 - 1:100 |
| Reactivities | Human, Mouse, Rat |
| Host | Rabbit |
| Isotype | IgG |
| Clonality | Polyclonal |
| Immunogen | C term -peptide of human PRKCA |
| Formulation | Store at -20C or -80C. Avoid freeze / thaw cycles. Buffer: PBS with 0.02% sodium azide, 50% glycerol, pH7.3 |
| Concentration | lot specific |
| Purification | Affinity purification |
| Conjugation | Unconjugated |
| Storage | Store at -20°C as received. |
| Stability | Stable for 12 months from date of receipt. |
| Predicted Protein Size | 77 kDa |
| Gene Name | protein kinase C alpha |
| Database Link | |
| Background | Activation of protein kinase C (PKC) is one of the earliest events in a cascade that controls a variety of cellular responses, including secretion, gene expression, proliferation, and muscle contraction . PKC isoforms belong to three groups based on calcium dependency and activators. Classical PKCs are calcium-dependent via their C2 domains and are activated by phosphatidylserine (PS), diacylglycerol (DAG), and phorbol esters (TPA, PMA) through their cysteine-rich C1 domains. Both novel and atypical PKCs are calcium-independent, but only novel PKCs are activated by PS, DAG, and phorbol esters. Members of these three PKC groups contain a pseudo-substrate or autoinhibitory domain that binds to substrate-binding sites in the catalytic domain to prevent activation in the absence of cofactors or activators. Control of PKC activity is regulated through three distinct phosphorylation events. Phosphorylation at Thr500 in the activation loop, the autophosphorylation site at Thr641, and at carboxy-terminal hydrophobic site Ser660 occurs in vivo. Atypical PKC isoforms lack hydrophobic region phosphorylation, which correlates with the presence of glutamic acid rather than the serine or threonine residues found in more typical PKC isoforms. Either the enzyme PDK1 or a close relative is responsible for PKC activation. A recent addition to the PKC superfamily is PKCµ (PKD), which is regulated by DAG and TPA through its C1 domain. PKD is distinguished by the presence of a PH domain and by its unique substrate recognition and Golgi localization. PKC-related kinases (PRK) lack the C1 domain and do not respond to DAG or phorbol esters. Phosphatidylinositol lipids activate PRKs and small Rho-family GTPases bind to the homology region 1 (HR1) to regulate PRK kinase activity. |
| Synonyms | AAG6; PKC-alpha; PKCA; PRKACA |
| Reference Data | |
| Protein Families | Druggable Genome, ES Cell Differentiation/IPS, Protein Kinase |
| Protein Pathways | Calcium signaling pathway, ErbB signaling pathway, Fc epsilon RI signaling pathway, Fc gamma R-mediated phagocytosis, Focal adhesion, Gap junction, Glioma, GnRH signaling pathway, Leukocyte transendothelial migration, Long-term depression, Long-term potentiation, MAPK signaling pathway, Melanogenesis, Natural killer cell mediated cytotoxicity, Non-small cell lung cancer, Pathogenic Escherichia coli infection, Pathways in cancer, Phosphatidylinositol signaling system, Tight junction, Vascular smooth muscle contraction, VEGF signaling pathway, Vibrio cholerae infection, Wnt signaling pathway |
Documents
| Product Manuals |
| FAQs |
| SDS |
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China