HSP90AB1 Rabbit Polyclonal Antibody
Frequently bought together (3)
Recombinant protein of human heat shock protein 90kDa alpha (cytosolic), class B member 1 (HSP90AB1)
USD 823.00
Transient overexpression lysate of heat shock protein 90kDa alpha (cytosolic), class B member 1 (HSP90AB1)
USD 436.00
Other products for "HSP90AB1"
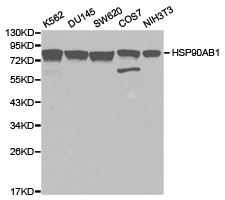
Specifications
| Product Data | |
| Applications | ELISA, IHC, WB |
| Recommended Dilution | WB,1:500 - 1:1000 IHC-P,1:50 - 1:200 ELISA,Recommended starting concentration is 1 μg/mL. Please optimize the concentration based on your specific assay requirements. |
| Reactivities | Human, Mouse, Rat |
| Host | Rabbit |
| Isotype | IgG |
| Clonality | Polyclonal |
| Formulation | Buffer: PBS with 0.02% sodium azide,50% glycerol,pH7.3. |
| Concentration | lot specific |
| Purification | Affinity purification |
| Conjugation | Unconjugated |
| Storage | Store at -20℃. Avoid freeze / thaw cycles. |
| Stability | Stable for 12 months from date of receipt. |
| Predicted Protein Size | 83kDa |
| Gene Name | heat shock protein 90kDa alpha family class B member 1 |
| Database Link | |
| Background | This gene encodes a member of the heat shock protein 90 family; these proteins are involved in signal transduction, protein folding and degradation and morphological evolution. This gene encodes the constitutive form of the cytosolic 90 kDa heat-shock protein and is thought to play a role in gastric apoptosis and inflammation. Alternative splicing results in multiple transcript variants. Pseudogenes have been identified on multiple chromosomes. |
| Synonyms | D6S182; HSP84; HSP90B; HSPC2; HSPCB |
| Reference Data | |
| Protein Families | Druggable Genome, Stem cell - Pluripotency |
| Protein Pathways | Antigen processing and presentation, NOD-like receptor signaling pathway, Pathways in cancer, Progesterone-mediated oocyte maturation, Prostate cancer |
Documents
| Product Manuals |
| FAQs |
| SDS |
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China