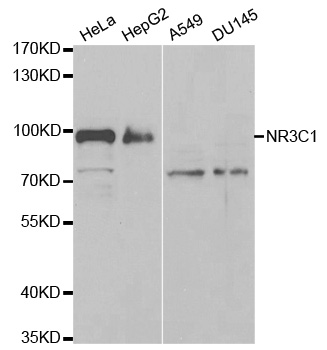
Glucocorticoid Receptor (NR3C1) Rabbit Polyclonal Antibody
Other products for "NR3C1"
Specifications
| Product Data | |
| Applications | IHC, WB |
| Recommended Dilution | WB 1:500 - 1:2000;IHC 1:50 - 1:200;IF 1:20 - 1:100 |
| Reactivities | Human, Mouse, Rat |
| Host | Rabbit |
| Isotype | IgG |
| Clonality | Polyclonal |
| Immunogen | Recombinant protein of human NR3C1 |
| Formulation | Store at -20C or -80C. Avoid freeze / thaw cycles. Buffer: PBS with 0.02% sodium azide, 50% glycerol, pH7.3 |
| Concentration | lot specific |
| Purification | Affinity purification |
| Conjugation | Unconjugated |
| Storage | Store at -20°C as received. |
| Stability | Stable for 12 months from date of receipt. |
| Predicted Protein Size | 86 kDa |
| Gene Name | nuclear receptor subfamily 3 group C member 1 |
| Database Link | |
| Background | Glucocorticoid hormones control cellular proliferation, inflammation, and metabolism through their association with the glucocorticoid receptor (GR)/NR3C1, a member of the nuclear hormone receptor superfamily of transcription factors . GR is composed of several conserved structural elements, including a carboxy-terminal ligand-binding domain (which also contains residues critical for receptor dimerization and hormone-dependent gene transactivation), a neighboring hinge region containing nuclear localization signals, a central zinc-finger-containing DNA-binding domain, and an amino-terminal variable region that participates in ligand-independent gene transcription. In the absence of hormone, a significant population of GR is localized to the cytoplasm in an inactive form via its association with regulatory chaperone proteins, such as HSP90, HSP70, and FKBP52. On hormone binding, GR is released from the chaperone complex and translocates to the nucleus as a dimer to associate with specific DNA sequences termed glucocorticoid response elements (GREs), thereby enhancing or repressing transcription of specific target genes . It was demonstrated that GR-mediated transcriptional activation is modulated by phosphorylation. Although GR can be basally phosphorylated in the absence of hormone, it becomes hyperphosphorylated upon binding receptor agonists. It has been suggested that hormone-dependent phosphorylation of GR may determine target promoter specificity, cofactor interaction, strength and duration of receptor signaling, receptor stability, and receptor subcellular localization . Indeed Ser211 of human GR is phosphorylated to a greater extent in the presence of hormone, and biochemical fractionation studies following hormone treatment indicate that Ser211-phosphorylated GR is found in the nucleus . Thus, Ser211 phosphorylation is a biomarker for activated GR in vivo. An added layer of complexity to GR signaling lies in the ability of multipl |
| Synonyms | GCCR; GCR; GCRST; GR; GRL |
| Reference Data | |
| Protein Families | Druggable Genome, Nuclear Hormone Receptor, Transcription Factors |
| Protein Pathways | Neuroactive ligand-receptor interaction |
Documents
| Product Manuals |
| FAQs |
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China