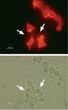
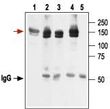
KCNH2 Rabbit Polyclonal Antibody
Other products for "KCNH2"
Specifications
| Product Data | |
| Applications | IF, IP, WB |
| Recommended Dilution | WB: 1:200-1:2000; IHC: 1:100-1:3000 |
| Reactivities | Human, Mouse, Rat |
| Host | Rabbit |
| Clonality | Polyclonal |
| Immunogen | GST fusion protein with sequence DSLSQVSQFMACEELPPGAPELPQEGPTRRLSLPGQLGALTSQPLHRHGSDPGS, corresponding to amino acid residues 1106-1159 of human Kv11.1 (HERG). Intracellular, C-terminus. |
| Formulation | Lyophilized. Concentration before lyophilization ~0.8mg/ml (lot dependent, please refer to CoA along with shipment for actual concentration). Buffer before lyophilization: Phosphate buffered saline (PBS), pH 7.4, 1 % BSA, 0.025% NaN3. |
| Purification | The serum was depleted of anti-GST antibodies by affinity chromatography on immobilized GST, and then anti-HERG antibody was affinity purified on immobilized HERG-GST. |
| Conjugation | Unconjugated |
| Storage | Store at -20°C as received. |
| Stability | Stable for 12 months from date of receipt. |
| Gene Name | potassium voltage-gated channel subfamily H member 2 |
| Database Link | |
| Background | The KV11.1 (HERG) channel is a member of the ether-a-go-go (EAG) subfamily of voltage-dependent K+ channels that includes the related proteins KV11.2 and KV11.3 (erg2 and erg3). KV11.1 possess the signature structure of the voltage-dependent K+ channels: six membrane-spanning domains and intracellular N and C termini. The KV11.1 current is characterized by strong inward rectification with slow activation and very rapid inactivation kinetics. The channel is expressed in the brain and heart (where it underlies the IKr current) and has a central role in mediating repolarization of action potentials. Mutations in the KV11.1 channel cause inherited long QT syndrome (LQTS) or abnormalities in the repolarization of the heart that are associated with life-threatening arrhythmias and sudden death. All the identified KV11.1 mutations produce loss of function of the channel via several cellular mechanisms ranging from alterations of gating properties, alterations of channel permeability/selectivity and alterations in intracellular channel trafficking that decreases the number of channels that reach the cell membrane. Lately drug-induced forms of LQTS have been reported for a wide range of non-cardiac drugs including antIHCistamines, psychoactive agents and antimicrobials. All these drugs potently block the KV11.1 channel as an unintended side effect, prompting regulatory drug agencies to issue recommendations for the testing of new drugs for their potential KV11.1 blocking effect. In addition, KV11.1 expression was found to be upregulated in several tumor cell lines of different histogenesis suggesting that it confers the cells some advantage in cell proliferation. Indeed, in several studies it has been shown that inhibition of the KV11.1 current leads to a decrease in tumor cell proliferation. Several toxins from scorpion venoms are potent blockers (affecting the channels in the nanomolar range) of KV11.1channels. Among these the most potent and selective are Ergtoxin-1, (16 nM) and BeKM-1 , (3 nM). In addition, the methanesulfonanilide class III antiarrhythmic agent E-4031, also blocks KV11.1 channel in the nanomolar range (7.7 nM). |
| Synonyms | ERG-1; ERG1; H-ERG; HERG; HERG1; Kv11.1; LQT2; SQT1 |
| Reference Data | |
| Protein Families | Druggable Genome, Ion Channels: Potassium, Transcription Factors, Transmembrane |
Documents
| Product Manuals |
| FAQs |
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China