HSP70-1A (HSPA1A) Rabbit Polyclonal Antibody
Frequently bought together (3)
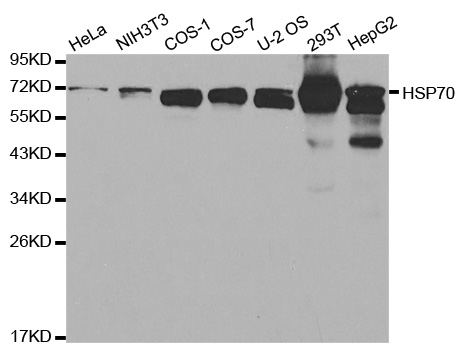
Transient overexpression lysate of heat shock 70kDa protein 1A (HSPA1A)
USD 396.00
Other products for "HSPA1A"
Specifications
| Product Data | |
| Applications | IF, WB |
| Recommended Dilution | WB 1:500 - 1:2000 |
| Reactivities | Human, Mouse |
| Host | Rabbit |
| Isotype | IgG |
| Clonality | Polyclonal |
| Immunogen | N term –peptide of human HSPA1A |
| Formulation | Store at -20°C (regular) and -80°C (long term). Avoid freeze / thaw cycles. Buffer: PBS with 0.02% sodium azide, 50% glycerol, pH7.3. |
| Concentration | lot specific |
| Purification | Affinity purification |
| Conjugation | Unconjugated |
| Storage | Store at -20°C as received. |
| Stability | Stable for 12 months from date of receipt. |
| Predicted Protein Size | 641 |
| Gene Name | heat shock protein family A (Hsp70) member 1A |
| Database Link | |
| Background | HSPA1A and HSP90 are molecular chaperones expressed constitutively under normal conditions to maintain protein homeostasis and are induced upon environmental stress (1). Both HSPA1A and HSP90 are able to interact with unfolded proteins to prevent irreversible aggregation and catalyze the refolding of their substrates in an ATP- and co-chaperone-dependent manner (1). HSPA1A has a broad range of substrates including newly synthesized and denatured proteins, while HSP90 tends to have a more limited subset of substrates, most of which are signaling molecules. HSPA1A and HSP90 often function collaboratively in a multi-chaperone system, which requires a minimal set of co-chaperones: HSP40, Hop, and p23 (2,3). The co-chaperones either regulate the intrinsic ATPase activity of the chaperones or recruit chaperones to specific substrates or subcellular compartments (1,4). When the ubiquitin ligase CHIP associates with the HSPA1A/HSP90 complex as a cofactor, the unfolded substrates are subjected to degradation by the proteasome (4). The biological functions of HSPA1A/HSP90 extend beyond their chaperone activity. They are essential for the maturation and inactivation of nuclear hormones and other signaling molecules (1,3). They also play a role in vesicle formation and protein trafficking (2). |
| Synonyms | HEL-S-103; HSP70-1; HSP70-1A; HSP70.1; HSP70I; HSP72; HSPA1 |
| Reference Data | |
| Protein Pathways | Antigen processing and presentation, Endocytosis, MAPK signaling pathway, Prion diseases, Spliceosome |
Documents
| Product Manuals |
| FAQs |
| SDS |
{0} Product Review(s)
0 Product Review(s)
Submit review
Be the first one to submit a review
Product Citations
*Delivery time may vary from web posted schedule. Occasional delays may occur due to unforeseen
complexities in the preparation of your product. International customers may expect an additional 1-2 weeks
in shipping.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China