Lentiviral Vectors as Catalysts for Vaccine Development
Jun 16, 2023

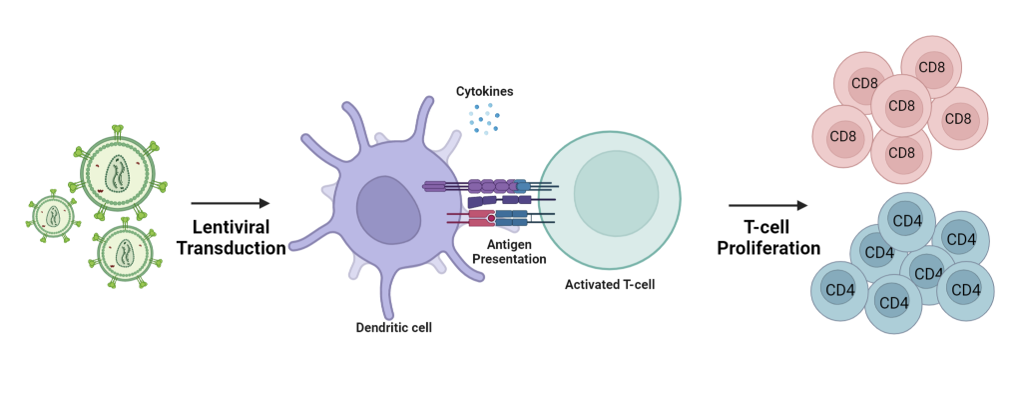
In the ever-evolving landscape of genetic research, lentiviral gene delivery has emerged as a powerful tool that has revolutionized the field of gene therapy and vaccine development. Lentiviral vectors (LVs), derived from HIV-1, have gained significant attention due to their unique ability to efficiently and stably transfer genetic material into both dividing and non-dividing cells. Particularly in the realms of infectious diseases and immuno-oncology, lentiviral vectors have gained substantial attention by effectively transducing dendritic cells (DCs) in vivo.
Recent studies have unveiled the ability of lentiviral vectors to induce endogenous gene expression of antigens within DCs, facilitating direct antigen presentation. This advantageous feature distinguishes lentiviral vectors from adenoviral vectors, eliminating the need for cross presentation and external antigen capture.
A study conducted in 2023 by Kirill Nemirov et al., demonstrated the remarkable capability of GFP lentiviral vectors, to transduce different subtypes of DCs in vivo, triggering the expansion of na?ve T cells2. These subtypes include:
- Myeloid
- CD11c+
- CD11b+
- CD8?
- Lymphoid
- CD11c+
- CD11b-
- CD8+
- Plasmacytoid
- CD11cint
- B220+
Excitingly, numerous clinical trials are currently underway, evaluating the therapeutic efficacy and safety of lentiviral-based gene therapy in the treatment of diseases such as ADA-SCID3?, as well as infectious diseases like Flavivirus, COVID-19, and Tuberculosis1.
Lentiviral Solutions for Researchers
OriGene proudly offers a range of cutting-edge lentiviral products, providing researchers with the tools they need to unlock the full potential of this revolutionary technology and make strides towards a healthier future.
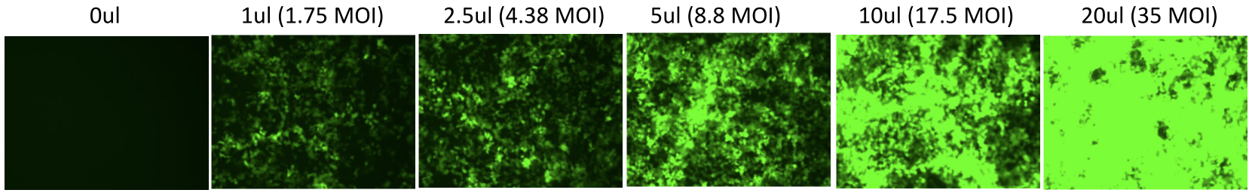
Lentiviral Validation Data
Download our Lentivirus 101 E-book
Related Products
Related Services
Citations
- Lundstrom K. Viral Vectors in Gene Therapy: Where Do We Stand in 2023? Viruses. 2023 Mar 7;15(3):698. doi: 10.3390/v15030698. PMID: 36992407; PMCID: PMC10059137.
- Nemirov K, Bourgine M, Anna F, Wei Y, Charneau P, Majlessi L. Lentiviral Vectors as a Vaccine Platform against Infectious Diseases. Pharmaceutics. 2023 Mar 5;15(3):846. doi: 10.3390/pharmaceutics15030846. PMID: 36986707; PMCID: PMC10053212.
- https://clinicaltrials.gov/ct2/show/NCT01380990






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China

